Human beings grow new brain cells throughout their late adult life, a new study shows, even continuing when people are approaching their 90s.
The findings, which reveal how long neurogenesis really extends in humans, also show the process is suspended in patients with Alzheimer's disease – a discovery that could help us explore new ways to treat the condition.
Most neurogenesis takes place during embryonic development, and by the time people are born, the majority of their neurons has already formed.
But that's not the end of the story. In the 1960s, it was first discovered that adult neurogenesis also occurs in mammals: new nervous system cells continue to grow in the brain, even as animals get older.
Exactly how this mechanism works in humans – and how long it lasts for – hasn't been easy to determine, due to a range of issues in studying human brains.
But in a new study led by molecular biologist Maria Llorens-Martin from Spain's Universidad Autónoma de Madrid, the researchers studied brain tissue from deceased human patients to examine adult hippocampal neurogenesis (AHN) in closer detail.
"Our research group is focused on investigating the mechanisms that control adult hippocampal neurogenesis, both under physiological and pathological conditions," the team's lab website explains.
"In particular, we are interested in determining the therapeutic potential of increasing adult hippocampal neurogenesis for the treatment of neurodegenerative diseases such as Alzheimer's disease (AD) and other tauopathies."
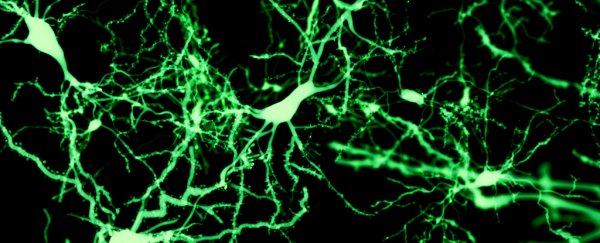
To find out whether new neurons continue to develop in older people, the researchers studied a region of the hippocampus called the dentate gyrus (DG) in tissue samples obtained from 13 deceased people.
These people, aged between 43 and 87 years old, had died for a range of reasons, including cancer, stroke, sepsis, and other fatal causes – but all were considered neurologically healthy before they passed away, leaving their bodies to science.
That final gesture reveals something important about adult neurogenesis: it extends until the ninth decade of life, the researchers say, and is shown by thousands of cells called doublecortin-expressing (DCX+) neurons in the DG, which correspond with neurogenesis.
"Altogether, these data strongly support the notion that subpopulations of DCX+ cells have a variable degree of maturation in the human DG," the authors write in their paper.
"The relative abundance of DCX+ immature neurons detected, together with expression of cell markers characteristic of both early and late stages of maturation, suggests that these cells also have an extended maturation period during AHN in humans."
When the team analysed the brains of 45 deceased Alzheimer's disease patients aged between 52 and 97 years, they noted "a marked and progressive decline in this number as the disease advanced".
In contrast, in neurologically healthy people, it looks like age brings a more moderate decline in adult neurogenesis, with the DG tissue of the 13 healthy patients showing a slighter decrease in DCX+ cells as their age ranged from 43 to 87.
"However, the number of DCX+ cells detected in neurologically healthy individuals of any age was consistently higher than that found in patients with AD, regardless of the age of these patients," the researchers explain.
"These data strongly support the notion that AD is a condition that differs from physiological ageing and suggest that, despite a physiological age-related decline in the population of DCX+ cells, independent neuropathological mechanisms contribute to devastating the population of immature neurons in AD."
The researchers also say the adult neurogenesis in AD patients looked to be negatively affected even in the early stages of the disease, before neurofibrillary tangles and senile plaques became pronounced.
While a lot more research needs to be done before we can understand why this is happening, the team proposes that a system to monitor early detection of AHN impairments by noninvasive methods could help doctors pick up the biomarkers of early AD before it progresses.
Making good on that hypothesis is work for the future, but right now we still walk away with something big.
"Our data bring to light the existence of a dynamic population of immature neurons in the human DG throughout physiological and pathological ageing until the tenth decade of life," the researchers conclude.
The findings are reported in Nature Medicine.