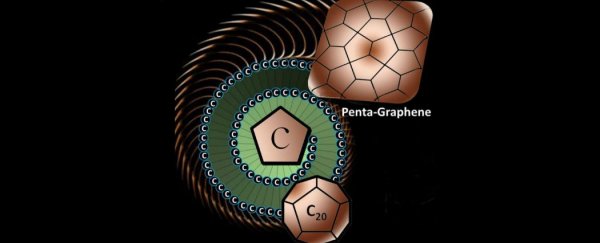
And international team of scientists has discovered penta- graphene, a super-thin sheet of pure carbon made up of perfect little pentagons.
Analysis of the material has shown that it's mechanically stable, so it has a good stiffness; it's thermally stable, which means it has a good amount of resistance to decomposition at high temperatures; and dynamically stable, so its structure won't undergo any changes without the influence of some kind of external force. And it has all kinds of weird and wonderful properties that the team says makes it a great candidate for applications in electronics, biomedicine, and nanotechnology.
"The three last important forms of carbon that have been discovered were fullerene, the nanotube, and graphene. Each one of them has unique structure," said lead researcher and US-based physicist Puru Jena, from the Virginia Commonwealth University (VCU), in a press release. "Penta-graphene will belong in that category."
Unlike most forms of carbon, which are made of hexagonal building blocks and the occasional pentagon, penta-graphene is made exclusively of pentagons. And the story behind how the researchers came to test out this new carbon structure is actually really sweet.
One of the team, Qian Wang, a professor at Peking University in China and an adjunct professor at VCU, was having dinner with her husband at a restaurant in Beijing, when she looked up at the walls and saw dozens of pentagon-shaped tiles, reminiscent of the ones that line the streets of Cairo in Egypt. "I told my husband, 'Come, see! This is a pattern composed only of pentagons,'" she said. "I took a picture and sent it to one of my students, and said, 'I think we can make this. It might be stable. But you must check it carefully.' He did, and it turned out that this structure is so beautiful, yet also very simple."
So they modelled the structure on a computer program and created a unique two-dimensional carbon allotrope made of pentagons. Simulations suggest that it's super-strong, and will not degrade at high temperatures - even up to 1,000 degrees Kelvin. "You know the saying, diamonds are forever? That's because it takes a lot of energy to convert diamond back into graphite," said Jena. "This will be similar."
Publishing in Proceedings of the National Academy of Sciences, the team reports that while graphene is a strong conductor of electricity, penta-graphene is semiconductor, which means it's not as good at carrying an electric current without dissipating a lot of power. But it manages to retain this property no matter how you bend and twist it.
"When you take graphene and roll it up, you make what is called a carbon nanotube, which can be metallic or semiconducting," says Jena. "Penta-graphene, when you roll it up, will also make a nanotube, but it is always semiconducting."
The team also found that penta-graphene stretches in really bizarre ways, thanks to a rare property known as Negative Poisson's Ratio. This means that the way its molecular bonds are hinged and oriented ensure that when it's stretched from either end, not only do those ends expends, but its width will also increase. So, it basically becomes fatter, not skinnier, when stretched at the end. Watch this for an example.
"If you stretch graphene, it will expand along the direction it is stretched, but contract along the perpendicular direction." said Wang. "However, if you stretch penta-graphene, it will expand in both directions."
The team is now going to work on synthesising a physical layer of penta-graphene, so they can start putting this new carbon variant to the test. "Once you make it, it [will be] very stable. So the question becomes, how do you make it?" says Jena. "We have some ideas. Right now, the project is theoretical. It's based on computer modelling, but we believe in this prediction quite strongly. And once you make it, it will open up an entirely new branch of carbon science. Two-dimensional carbon made completely of pentagons has never been known."