Ice is a complex beast. While you and I only ever come into contact with one kind of it, scientists actually know of around 20 different molecular varieties – some so esoteric and rare, they may only exist inside computer simulations, or buried within distant planets.
But just because water can freeze into a solid in so many different ways, its crystallisation is not necessarily inevitable. In a new experiment, scientists were able to create water that doesn't seem to ever turn into ice, even when subjected to temperatures approaching absolute zero.
There are a number of ways of developing so-called 'unfreezable water', but scientists are continually finding new approaches to test the limits of these techniques.
Some of these involve the study of what's known as amorphous ice, an amorphous yet solid form of water, where ice never forms because water molecules are prevented from the process of crystallisation.
Last year, scientists in Sweden succeeded in supercooling liquid water to a record-low temperature of around –45 degrees Celsius (–49 degrees Fahrenheit) without ice forming, but now researchers in Switzerland have gone even further.
Using a form of 'nanoconfinement' of water molecules with the help of synthesised lipid-based membranes, researchers from ETH Zurich and the University of Zurich were able to take water all the way down to –263 degrees Celsius (–441 degrees Fahrenheit), just 10 degrees Celsius above absolute zero, without it becoming ice.
"The physical confinement of water at the nanoscale can play a major role in controlling its properties," the authors explain in their paper.
"In particular, confinement in the nanometre range can inhibit the arrangement of water molecules into an ice structure, and thereby prevent crystallisation at subzero temperature and create a state of amorphous water."
To trap their unsuspecting water molecules, the team synthesised a new class of fat molecules that form into a soft biological material called a lipidic mesophase.
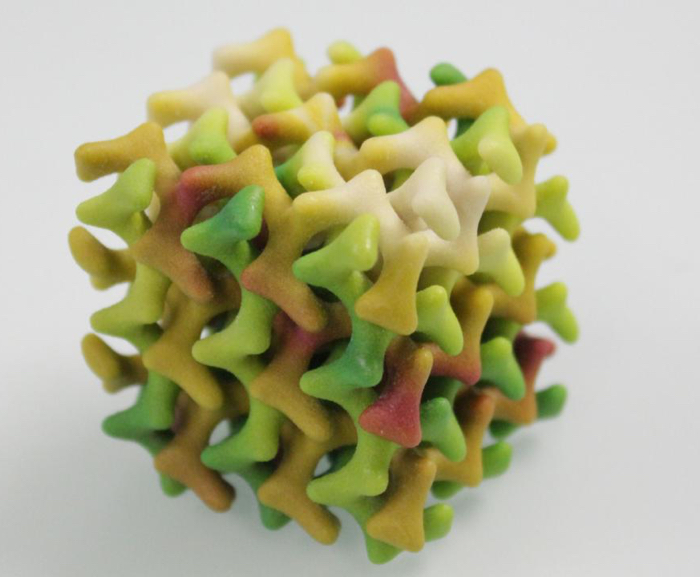 3D model of the lipid mesophase (Peter Rüegg/ETH Zurich)
3D model of the lipid mesophase (Peter Rüegg/ETH Zurich)
In this structure, the fat molecules self-assemble into an arrangement that creates an extremely narrow network of connected channels, each measuring less than 1 nanometre in diameter.
Cramped inside those tiny tunnels, water molecules can exist in liquid form, but the space is so incredibly confined, ice crystallisation is impossible at the molecular level – even when the lipidic mesophase was cooled with liquid helium down to around 10 Kelvin.
This nanoconfinement feat, the researchers say, is due to molecular modifications inside the atomic structure of the fatty material, which rearranges hydrophobic (water-repelling) and hydrophilic (water-attracting) parts of the mesophase.
"The novelty of our lipids is the introduction of highly strained three-membered rings into specific positions within the hydrophobic parts of the molecules", says one of the team, chemist Ehud Landau from the University of Zurich.
"These enable the necessary curvature to produce such tiny water channels and prevent lipids to crystallise."
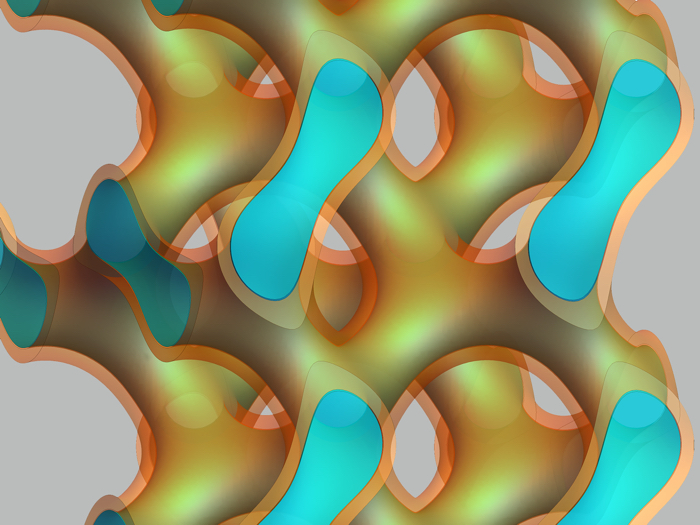 The material forms branched membranes that confine water, seen in light blue (Livia Salvati Manni/ETH Zurich)
The material forms branched membranes that confine water, seen in light blue (Livia Salvati Manni/ETH Zurich)
While crystallisation doesn't occur, at these super-low temperatures, the water does become 'glassy': a sub-state of matter that is solid but nonetheless not ice, given at the molecular level, it is not crystalline the way ice is.
Given ice crystals never form, the researchers say the lipid mesophase membranes could one day help scientists preserve protein-based matter without damaging it, keeping it in its original form at very low temperatures, or help us figure out new ways of preventing water from freezing into ice.
"But our work wasn't aimed at exotic applications," says physicist and soft materials researcher Raffaele Mezzenga from ETH Zurich.
"Our main focus was to give researchers a new tool to facilitate the study of molecular structures at low temperature without ice-interfering crystals, and ultimately to understand how two main components of life, water and lipids, interact under extreme conditions of temperature and geometrical confinement."
The findings are reported in Nature Nanotechnology.
