New research has traced the history of antibiotics and antibiotic resistance back some 350-500 million years, a study in deep microorganism genealogy that could help in our current battle to contain the rise of superbugs.
The team focussed on a class of antibiotics called glycopeptides - such as vancomycin and teicoplanin - and the way they have evolved down the millennia. Vancomycin is defined as an essential medicine by the World Health Organisation, and can be used as a last resort medication in some cases.
These antibiotics are also under a growing threat from antibiotic resistance, as bacteria evolve to resist the best medicines we can throw at them. The researchers hoped to uncover some clues as to how we might win the evolutionary race in the future, and outpace the defences that microbes are putting up.
"The results we uncovered in this study offer a valuable lens through which to consider the current antibiotic crisis," says biochemist Nicholas Waglechner, from McMaster University in Canada.
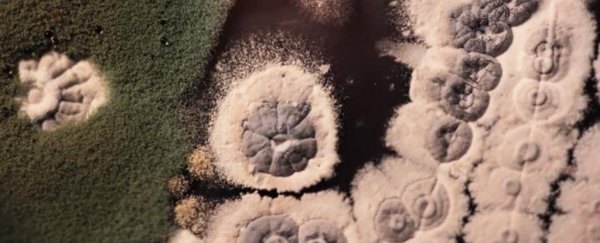
Tracking glycopeptides back through time isn't easy. As the team notes in their paper, glycopeptide antibiotics are produced by Actinobacteria, an important phylum of microbes found in soil.
To track the synthesis of glycopeptide antibiotics through evolutionary time, the researchers had to turn to biosynthetic gene clusters (BGCs) that can contain genes not just for producing and regulating these glycopeptides, but also for resistance. Not an easy task, considering BGCs can vary in their chemical make-up, and can each have their own separate family tree.
"Extracting this history from genome sequences is difficult as conservation of the individual components of these gene clusters is variable and each component can have a different trajectory," the team writes in their study.
This is one of only a few studies so far that's used BGCs to look at how antibiotic production and antibiotic resistance might have evolved in tandem. Some of the biosynthesis that has happened along the way could be useful in engineering future drug treatments.
The researchers found that glycopeptide resistance sprang up in Actinobacteria around the same time as the genes responsible for producing the ancestors of vancomycin – and all that happened around 350 to 500 million years ago.
"These compounds have been useful to bacteria on the planet even before dinosaurs appeared, and resistance co-evolved with production as a means of self-protection for producing bacteria," says Waglechner.
"The use of vancomycin in modern times in medicine and agriculture has resulted in the movement of resistance from these innocuous producers to disease-causing bacteria over a few short decades."
If we are able to produce improved medications from learning about this evolutionary context, the sooner it arrives the better. Evidence of antibiotic resistance is now being found in some of the remotest places on Earth, and even in space.
All hope is not lost though. Scientists continue to discover new possibilities for developing the next wave of antibiotics, whether that's looking in the soil microbes clinging to insects, or combining existing drugs together in new ways.
"Our findings are of significant interest," says biochemist Gerry Wright, from McMaster University in Canada.
"Our study reveals several implications in how we could potentially manage antibiotic use and find new drugs for antimicrobial infections."
The research has been published in Nature Microbiology.