In nuclear physics, certain elements or their isotopes can be classified as 'magic', based on a specific magic number of protons or neutrons found in their atomic nuclei.
The seven most widely recognised magic numbers are 2, 8, 20, 28, 50, 82, and 126, and while they obviously don't impart any special supernatural powers (damn), they do render the nucleus far more stable and strongly bound than other, non-magic nuclei.
To understand magic numbers, you need to wrap your head around the nuclear shell model - one of a number of models used to describe the composition of a nucleus. Basically, the nucleus shell model describes the nucleus it as if it were behaving like electrons in shells around an atom - described by the atomic shell model.
So you've got an atom, with negatively charged electrons spinning around its nucleus in 'shells' - layers of well-defined energy levels - and if a shell is 'full', it results in greater stability for the atom.
Inside the nucleus, you've got a bunch of positively charged protons and neutral neutrons all milling about, and that in itself should be a very big problem for the atom.
Seeing as protons all have an identical charge, which causes them to electromagnetically repel each other whenever they get too close, what's stopping them from bursting right through the nucleus and causing the whole thing to self-destruct?
That's where the strong nuclear force - one of the four basic forces of nature, the others being gravity, the electromagnetic force, and the weak nuclear force - comes in, because it's stronger than the force of repelled protons, and this keeps the nucleus from breaking apart.
But just like you can apply enough energy to an atom to kick an electron out, you can do the same thing with protons and neutrons, and the amount of energy you need is dependent on the element, whether you're looking at helium at one end of the periodic table, or uranium at the other.
These different energy levels suggest that the protons and neutrons are being held in defined 'shells' in the nucleus - like electrons in an atom - and when those shells are complete, they require a whole lot more energy to kick a proton or a neutron out.
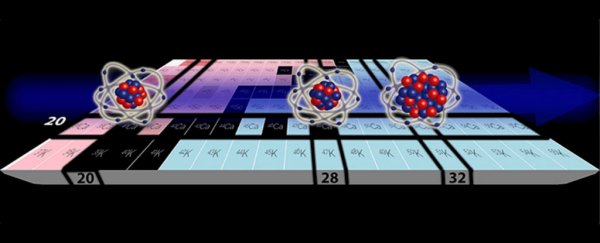
According to the nuclear shell model, when all the protons or neutrons in a nucleus are in filled shells, the number of protons or neutrons is called a magic number, for example, tin (Sn) has a magic number of 50 protons, and iron (Fe) has a magic number of 28 neutrons.
Some nuclei have magic numbers of both protons and neutrons, and are known for their exceptional stability. These nuclei are classified as 'doubly magic'.
Calcium with the magic proton number of 20 can be doubly magic when it has 20 or 28 neutrons, and that gives the Calcium-40 and Calium-48 isotopes doubly magic nuclei.
But experimental evidence has suggested that there could be more magic numbers out there. A team led by physicist Gaute Hagen from the US Department of Energy's Oak Ridge National Laboratory was investigating the calcium-48 isotope, and unexpected results prompted them to question long-standing assumptions about what makes a doubly magic nucleus.
They decided to look at the larger, heavier, and more complex isotope, Calcium-52, to see if they could expand on the known magic numbers.
They found through several models and simulations that it had a higher excitation energy and larger neutron separation energy than thought, the implications of which were pretty mind-blowing. "These quantities tell us something about how strongly a nucleus is bound, and therefore led researchers to believe that calcium-52, much like calcium-48, was magic," says Hagen.
Now, the team has come back to test this hypothesis, by measuring the radius of the nucleus when certain numbers of neutrons were added.
"The previously known doubly-magic isotopes, 40Ca and 48Ca, have similar and smaller charge radii than their neighbours because they are particularly strongly bound," one of the team, Ronald Fernando Garcia Ruiz, said in a press statement.
"When we measured charge radii for larger neutron numbers, they kept growing. Based on observations from other doubly-magic nuclei, we would have expected a relative drop in the charge radius if 52Ca were doubly-magic, too. However, it increases as other non-magic nuclei in this region of the nuclear chart," he adds.
What doesn't make sense is that several nuclear models did not predict the radius of Calcium-58 to grow as much as it did in the experiment, and the team can't explain it using any aspect of our current understanding of nuclear physics.
"Theory before this was happy, because it described other aspects of neutron-rich calcium isotopes," says Garcia Ruiz. "But all of the theoretical models employed underestimated this growth in charge radii. We've shown there are missing components in our knowledge."
So the magic number of seven magic numbers is safe for now, but it's been accompanied by a nice big elephant in the room - do we really understand how magic nuclei work at all?
The team now plans to complete further testing using the Oak Ridge National Laboratory's new Summit supercomputer, which will hopefully give them far more precision to work with.
"Our results for this simulation really point us back to the fundamental interaction that we're studying," said one the team, Gustav Jansen. "It's saying that we need to do a better job of approximating this interaction, but also we need to narrow down the associated uncertainties."
The results have been published in Nature Physics.