Oganesson (Og) is the heaviest chemical element in the periodic table, but its properties have proved difficult to measure since it was first synthesised in 2002.
Now an advanced computer simulation has filled in some of the gaps, and it turns out the element is even weirder than many expected.
At the atomic level, oganesson behaves remarkably differently to lighter elements in several key ways – and that could provide some fundamental insights into the basics of how these superheavy elements work.
The simulations run by the international team of scientists show that oganesson's electrons, protons, and neutrons don't follow the same rules as the other noble gases that the element is grouped with, and that could have a big impact on how we understand this section of the periodic table.
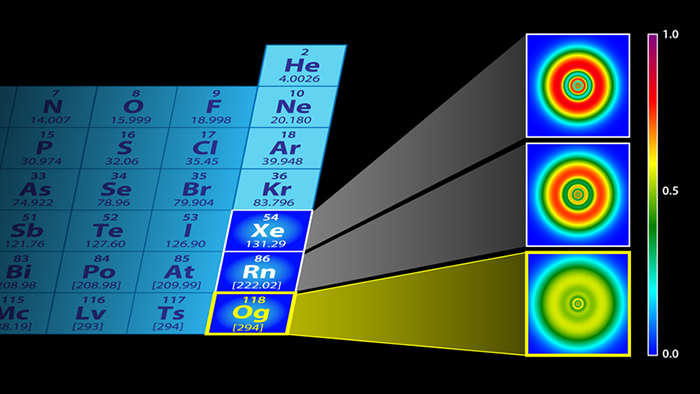 Electronic structures of xenon (top), radon (middle), and oganesson (bottom). (P. Jerabek et al and APS/Alan Stonebraker)
Electronic structures of xenon (top), radon (middle), and oganesson (bottom). (P. Jerabek et al and APS/Alan Stonebraker)
"The superheavy elements represent the limit of nuclear mass and charge," says one of the researchers, Witek Nazarewicz from Michigan State University. "They inhabit the remote corner of the nuclear landscape whose extent is unknown."
"The questions pertaining to superheavy systems are in the forefront of nuclear and atomic physics, and chemistry research."
In lighter elements in the same noble gas family as oganesson, according to the Bohr model of the atom, electrons take up certain orbits or positions around the nucleus, forming shell-like groups around the centre.
Calculations known as fermion localisation functions are used to work out where these electron shells are, but such are the large electrostatic forces produced by an oganesson atom, the rules of special relativity come into play.
With that in mind, the researchers used adapted fermion localisation functions called electron localisation functions to calculate where the electrons would be in oganesson. Turns out, the electron shells become almost indistinguishable, creating a kind of electron gas around the nucleus.
In other words, at the most fundamental level, it's not like other noble gases such as xenon or neon at all.
"On paper, we thought that it would have the same rare gas structure as the others in this family," says one of the researchers, Peter Schwerdtfeger from Massey University in New Zealand.
"In our calculations however, we predict that oganesson more or less loses its shell structure and becomes a smear of electrons."
That same smear or special gas state also applies to the neutrons inside the superheavy nucleus, according to the researchers' calculations, though the protons were shown to retain some kind of shell-like status.
We're talking some deep-level quantum physics here, but what it all means is that oganesson doesn't seem to be like the other elements it's grouped with. The special blob formation of its electrons could mean it's much more chemically reactive than the other noble gases, for example.
Another possible consequence is that oganesson atoms would clump together in a solid at room temperature, rather than bouncing off one another as they would usually in a gas.
Now bear in mind these are just computer simulations, albeit very complex ones – they aren't studies of oganesson itself. The element is too hard to produce and lasts for such a short time that we can't really examine it in the usual ways.
But now we have these predictions about the structure and properties of element 118, scientists can put together experiments to try and put these hypotheses to the test. That's the next stage in the research.
Further down the line, these insights could even help us work out how to produce an oganesson atom that lasts for more than a millisecond.
"Calculations are the only way to get at [oganesson's] behaviour with the tools that we currently have, and they have certainly provided some interesting findings," says Schwerdtfeger.
The research has been published in Physical Review Letters.