Set in the middle of a mass of laser equipment, researchers have managed to trigger the coldest chemical reaction in the known Universe. This feat promises to reveal some essential truths about how the building blocks of matter react at ultra-low temperatures.
How cold is the reaction exactly? We're talking in the region of 500 nanokelvin - just a few millionths of a degree above absolute zero. The frigid nature of this set-up is important, since at these sort of temperatures molecules tend to slow to the point of almost stopping.
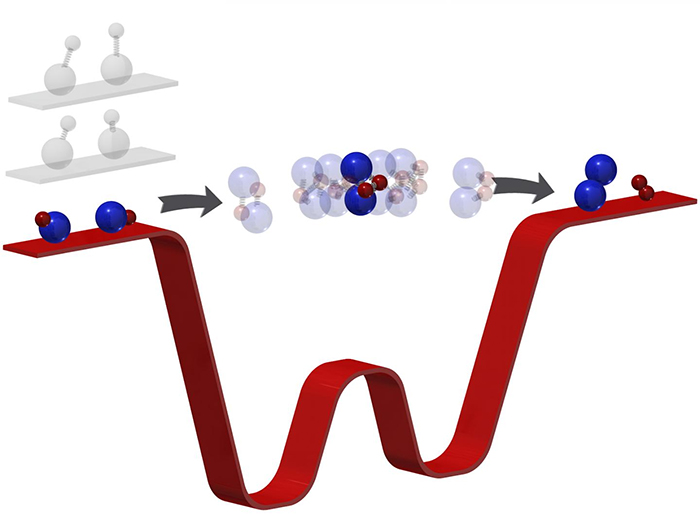
If you want a chemical reaction to happen, tardy molecules are not what you'd typically be after. But in this case, the reduction in both temperature and speed gave the Harvard University-led team the opportunity to see something that's never been observed before: the moment when two molecules meet together and form… two new molecules.
 Scientists have been able to observe the central part of a chemical reaction. (Ming-Guang Hu)
Scientists have been able to observe the central part of a chemical reaction. (Ming-Guang Hu)
"Probably in the next couple of years, we are the only lab that can do this," says physicist Ming-Guang Hu, from Harvard University.
Chemical reactions take just a picosecond, which makes trying to capture what happens in that time frame very tricky indeed. Even ultra-fast lasers acting as cameras can usually capture the start and end of a reaction, not what happens in the middle.
Slowing the reaction in the extremely cold temperatures achieved by the team was therefore the perfect solution.
"Because [the molecules] are so cold, now we kind of have a bottleneck effect," says chemical biologist Kang-Kuen Ni, also from Harvard University.
The absolute coldest temperature in the Universe is absolute zero - but it's impossible to achieve, because it means atoms would stop completely. We can, however, get close to it.
Ultra-low temperatures mean ultra-low energy, which in turn means a much slower reaction: two potassium rubidium molecules chosen for their pliability were delayed in the reaction stage for microseconds (millionths of a second).
A technique known as photoionisation detection was then used to observe what was happening to the two molecules, giving scientists invaluable real data to help inform their models and hypotheses.
Being able to observe chemical reactions at such close quarters and at such a fundamental level opens up the possibility of being able to design new reactions too – an almost limitless number of combinations are imaginable, potentially useful in everything from material construction to quantum computing.
It's a journey that Kang-Kuen Ni has been on for years – working at incredibly small scales to observe and to control what happens when chemicals react with each other.
Now the team is investigating ways in which chemical reactions could be influenced or manipulated to order – either changing the energies involved before the reaction happens, or even nudging the molecules to alter the reaction while it's in progress.
"With our controllability, this time window is long enough, we can probe," says Hu. "Now, with this apparatus, we can think about [influencing reactions]. Without this technique, without this paper, we cannot even think about this."
The research is published in Science.
