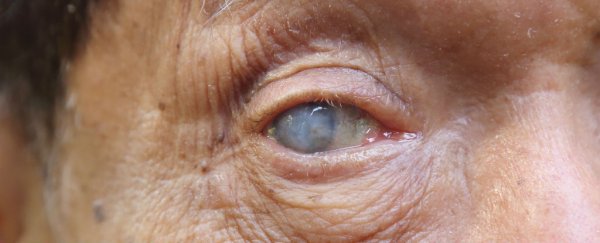
Three women in Florida have spent US$5,000 each to take part in a clinical trial of an unproven stem cell treatment to address their macular degeneration - a progressive eye disease that leads to retina damage and vision loss.
Two of the women thought they were participating in a government research project, but it became clear that this trial was not only incredibly dodgy and unscientific, it was so dangerous, it left them blind.
"There's a lot of hope for stem cells, and these types of clinics appeal to patients desperate for care who hope that stem cells are going to be the answer, but in this case these women participated in a clinical enterprise that was off-the-charts dangerous," says Thomas Albini from the University of Miami.
Albini was not involved with the trial, but treated the women once things took a turn for the worse, and co-wrote a case report on the incident for The New England Journal of Medicine.
"We expect health care providers to take every precaution to ensure patient safety," he says, "but this definitely shows that the lack of oversight can lead to bad players and bad outcomes. It's alarming."
According to a new report of the incident, the women, aged 72 to 88, paid thousands of dollars each to have fat removed from their bodies via liposuction, which was then used to extract what's known as adipose-derived stem cells and plasma.
A stem cell and plasma solution was then injected to both eyes of each patient, which is already setting off alarm bells, because experimental treatments should only ever be tested in one eye due to the potential side effects.
And what side effects they were.
Within a week of undergoing treatment, the women experienced vision loss, detached retinas, and bleeding from the eyes, and now a year on, remain totally blind.
Albini now says it's "extremely unlikely" they'll ever see again.
While the macular degeneration they were trying to treat tends to result in blurred vision and sometimes blindness, there are treatments that can slow down the progression of the disease, and patients can live for many years experiencing only gradual changes in vision.
Considering these three women still had their vision when they signed up for the clinical trial, the results have left the them with a far worse fate than if they had treated it using conventional methods.
According to Reuters, an estimated 600 clinics around the US are currently claiming to use stem cells to treat a wide variety of disorders, and some are doing so without basic safety testing and scientific evidence.
What makes this is so dangerous is that stem cell treatments are actually an extremely promising avenue of medical research, and members of the public can be tricked into thinking that they too will see the amazing results that have been reported over the past couple of years.
Just last year, researchers reported that stem cell injections enabled stroke patients to walk again, and in 2015, they halted the progression of Multiple sclerosis (MS) by 91 percent.
Back in January, researchers used stem cells to get damaged teeth to repair themselves, and later this year, a new clinical trial is expected to test an experimental stem cell treatment that appears to be able to repair any tissue in the body.
We're not talking fringe science here, and the fact that the clinical trial in Florida was listed on ClinicalTrials.gov, a registry and results database run by the US National Library of Medicine, made it appear way more legit than it was.
"There is a lot of very well-founded evidence for the positive potential of stem therapy for many human diseases, but there's no excuse for not designing a trial properly and basing it on preclinical research," says Jeffrey Goldberg, head of ophthalmology at the Stanford University School of Medicine, and co-author of the report.
So how is any of this even legal?
As George Dvorsky points out over at Gizmodo, the trial did not need to gain FDA approval because the stem cells weren't being transferred between patients, and were considered to be "minimally processed".
"The FDA has since revised its requirements, and it now needs approval for these types of procedures," Dvorsky reports. "In addition to updating its regulations, the FDA is also clamping down on stem cell clinics."
While the name of the clinical involved has not been disclosed, it was sponsored by the Florida-based Bioheart Inc., now known as US Stem Cell Inc.
In response to the case study being published, the US Stem Cell Inc. says it no longer runs such trials, telling Gizmodo, "Neither US Stem Cell nor US Stem Cell Clinic currently treats eye patients."
While all of this is too late to save the three women who now have to live out their days in darkness, it does serve as a timely warning for anyone else seeking stem cell treatments.
As Gene Emery reports for Reuters, warning signs that something was amiss in this trial include the fact that the doctors giving the injections were not eye specialists, and the study group was not affiliated with an academic medical centre.
It's also odd that the patients had to pay to be involved - something that is never done in a major research study, says Emery.
"I'm not aware of any legitimate research, at least in ophthalmology, that is patient-funded," Albini adds in a statement.
If you are ever in the market for stem cell treatments, other than checking if trial is affiliated with an academic medical centre, the researchers advise consult the A Closer Look at Stem Cells website, which is maintained by the International Society for Stem Cell Research.
The case study has been published in The New England Journal of Medicine.