For the first time, scientists have used cellular reprogramming to reverse the ageing process in living animals, enabling mice with a form of premature ageing to live 30 percent longer than control animals.
The technique involves the use of induced pluripotent stem cells ( iPSCs), which lets scientists reprogram skin cells to a base, embryonic-like state. From there, iPSCs can develop into other types of cells in the body – and now researchers have shown that reprogramming cells can also rejuvenate living creatures, in addition to winding back cells.
"In other studies scientists have completely reprogrammed cells all the way back to a stem-cell-like state," says researcher Pradeep Reddy from the Salk Institute for Biological Studies.
"But we show, for the first time, that by expressing these factors for a short duration, you can maintain the cell's identity while reversing age-associated hallmarks."

The iPSC technique was developed by Japanese researcher Shinya Yamanaka in 2006, when he discovered that differentiated cells could be wound back to embryonic-like stem cells by inducing the expression of four genes now known as the Yamanaka factors.
But while reprogramming cells to such an embryonic-like state sounds like it might make organisms younger, it also introduces dangerous complications. Research in 2013 and 2014 found that introducing iPSCs in living animals was fatal, resulting in cancerous growths or organ failure from adult cells having lost their identity.
"Obviously there is a logic to it," epigenetics researcher Wolf Reik from the University of Cambridge in the UK, who wasn't involved with the study, told Hannah Devlin at The Guardian.
"In iPS cells you reset the ageing clock and go back to zero. Going back to zero, to an embryonic state, is probably not what you want, so you ask: where do you want to go back to?"
That kind of thinking led the Salk researchers to attempt partial reprogramming. Rather than inducing the expression of the Yamanaka factors for up to three weeks – which leads to pluripotency – they only induced the genes for two to four days.
This means the cell retains its differentiation – ie. a skin cell stays a skin cell, not being wound back all the way to a stem cell – but it effectively becomes a younger version of itself.
At least, that's the hypothesis, and the researchers suspect that partial reprogramming removes the build-up of what's called epigenetic marks in our cells – the wear and tear that builds up in our genome in response to environmental and external factors.
Over time, these marks become more and more pronounced, degrading cell efficiency and contributing to what we experience as ageing. The researchers liken the process to a manuscript that's become illegible due to too many hand-written edits.
"At the end of life there are many marks and it is difficult for the cell to read them," one of the team, Izpisua Belmonte, told Nicholas Wade at The New York Times.
While that remains a hypothesis for now, the researchers' experiments suggest they're onto something.
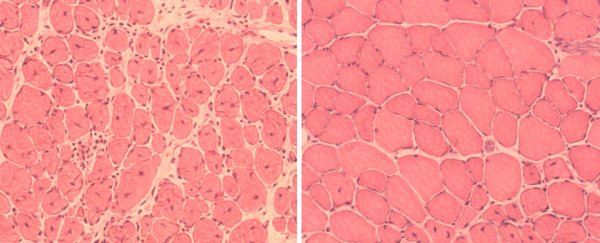
In mice with progeria – a rare genetic disease that brings about premature ageing – animals that received a partial reprogramming treatment lived for 24 weeks on average, while untreated animals with the same illness lived for just 18 weeks.
"It is difficult to say specifically why the animal lives longer," one of the team, Paloma Martinez-Redondo says in a press release.
"But we know that the expression of these factors is inducing changes in the epigenome, and those are leading to benefits at the cellular and organismal level."
In addition to a longer lifespan, the treated animals' health also received a boost, with the mice showing improved cardiovascular and organ functions.
When the treatment was applied to healthy mice without progeria, they too showed improved organ health – but it's too early to say whether their longevity was also affected, as the animals are still living.
While these results are promising, it's still early days for this research – especially to the extent that it could one day be applied to humans.
We've only seen these results in mice so far, but the researchers are hopeful that a selective inducement of the Yamanaka factors might produce similar effects in people.
"Obviously, mice are not humans and we know it will be much more complex to rejuvenate a person," says Belmonte.
"But this study shows that ageing is a very dynamic and plastic process, and therefore will be more amenable to therapeutic interventions than what we previously thought."
The team now intends to look into the development of molecules that may be able to mimic the Yamanaka factors, with a focus on the rejuvenation of specific tissues and organs.
These medicines won't be available tomorrow, but on the other hand, it doesn't sound like they're too far away either.
"These chemicals could be administrated in creams or injections to rejuvenate skin, muscle, or bones," Belmonte told The Guardian.
"We think these chemical approaches might be in human clinical trials in the next 10 years."
The findings are reported in Cell.