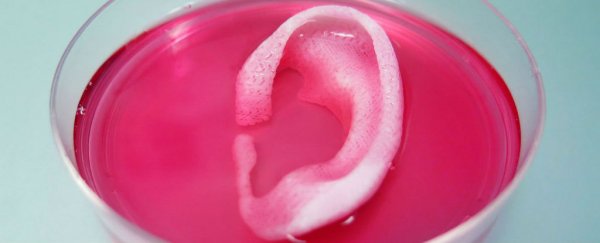
For the first time, scientists have used a 3D printer to produce life-sized body parts and tissues using living cells as the 'ink'. Not only are these structures large and sturdy enough to be a viable replacement for the real thing - something that previous bioprinters have failed to do - they're personalised and functional, not a 'one size fits all' cosmetic add-on.
"It can fabricate stable, human-scale tissue of any shape. With further development, this technology could potentially be used to print living tissue and organ structures for surgical implantation," one of the researchers behind the technology, Anthony Atala from the Wake Forest Institute for Regenerative Medicine, told The Guardian.
While bioprinters have been used to print miniature or more simplistic replicas of organs - including brains and kidney tissues - so scientists can carry out research on them rather than on real ones (lab animals everywhere, rejoice), until now, no one's been able to print something large, stable, and 'alive' enough to act as a reliable transplant.
One of the biggest hurdles has been in figuring out how to keep those cells alive through the printing process, and how to build structures that incorporate all the things that keep our organs running, such as blood vessels and vascular structures to maintain oxygen flow.
"Cells simply cannot survive without a blood vessel supply that's smaller than 200 microns [around 0.1 cm], which is extremely small," Atala told Gizmodo, adding that this has been the limiting factor for bioprinters in the past. "That's the maximum distance. And that's not just for printing, that's nature."
Atala and his team figured out how to overcome this by combining living cells extracted from transplant recipients with special types of plastics and gels that have been designed to mimic biological tissues, muscle, and cartilage. These materials provide the structure the 3D-printed body parts need while they're surgically implanted, and once in place, the plastic and gel components fade away, leaving only biological materials.
"At the same time, the cells secrete a supporting matrix that helps maintain the implant's shape," Arielle Duhaime-Ross explains at The Verge. "By the end of this process, the cells have reorganised themselves in a self-sufficient manner that negates the need for supporting materials."
So once these structures are implanted, they shed their artificial scaffolding, and then encourage the growth of living supports from the recipient's body, such as new tissue, bone, or cartilage cells.
The researchers demonstrated their technology by making ear, bone, and muscle structures using living cells extracted from humans, rabbits, mice, and rats. They're yet to test the implants on humans, but when they implanted human-sized ears under the skin of mice (yep, those poor mice), the ears retained their shape, grew new supporting cartilage, and maintained a healthy blood supply within two months.
Two weeks after the rats received 3D-printed muscle tissue, nerve cells started growing around it, and in a five-month trial, skull fragments implanted into rats had formed new bone tissue with a functioning blood supply.
It's still early days for the technology - and will be "early days" until the team can prove that it works in human trials - but things are looking promising. As Adam Feinberg, a biomedical engineer at Carnegie Mellon University who wasn't involved in the study, told The Verge: "You're going to see a lot of exciting advances over the next year or two that will push this from the realm of science fiction into something that's close to impacting patients."
The research has been published in Nature Biotechnology.