Three years after clinical trials in humans first began, researchers in the US have shown that their bionic eye device isn't only safe to use, it also reliably restores vision in those who suffer from the degenerative eye disease retinitis pigmentosa.
The results found that 89 percent of the subjects involved in the trial could see significantly better after using the device, which is known as Argus II, and 80 percent had their quality of life improved. It's an exciting result, as the device works in a similar way to the cochlear implant - or 'bionic ear' - which has helped restore hearing to hundreds of thousands of people.
"This study shows that the Argus II system is a viable treatment option for people profoundly blind due to retinitis pigmentosa," lead researcher Allen C. Ho from the Wills Eye Hospital in Pennsylvania, US, said in a press release. "One that can make a meaningful difference in their lives and provides a benefit that can last over time."
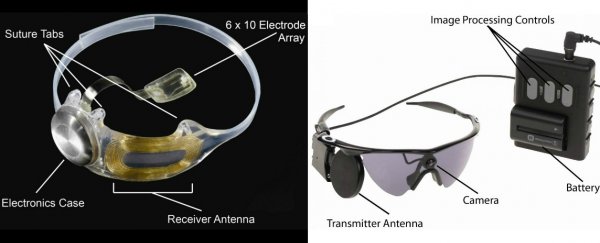
The device was created by medical company, Second Sight, and works through an electronic device that's implanted onto a patient's retina - the layer of light-sensing cells at the back of the eye.
To stimulate these cells, visual information is captured by a tiny camera attached to the patient's glasses and sent to a pocket-sized computer, where it's processed into electronic signals. These signals are beamed wirelessly to the patient's implant, which stimulates the retinal cells with painless electrical pulses. The result is that the patient sees patterns of light, that the brain can eventually learn to interpret as an image.
The Argus II received limited Food and Drug Administration (FDA) approval in 2013, but this clinical trial set out to show that the device could be used more broadly by testing it over three years. They worked with 30 patients aged between 28 and 77 who had little or no light perception in both eyes as a result of retinitis pigmentosa.
The team tested their visual function in the lab and in real-world conditions over the next three years. The results were overwhelmingly positive, with independent investigators finding that no subjects had been affected negatively by the bionic eye, and 80 percent had both their vision and quality of life significantly improved.
There were no device failures during the trial, the researchers report, and only 11 adverse events, most of which arose shortly after surgery and were successfully treated. Only one device had to be removed after it became damaged and began to erode.
The team now hopes that the results, which have been published in the journal Ophthalmology, will allow them to begin testing the device in an even broader range of subjects, with the aim of eventually using the device to restore sight to more patients.
"I look forward to future studies with this technology which may make possible expansion of the intended use of the device, including treatment for other diseases and eye injuries," said Ho.
We can't wait.