After decades of research, scientists think they finally know what turns prions - healthy proteins inside our brains - into the infectious, virus-like pathogens that cause 'mad cow' disease, and have also been linked to Alzheimer's and Parkinson's.
It turns out, copper ions might be part of the problem.
If you haven't heard of prions before, be prepared to have your mind exploded by how creepy they are.
Although they start out as harmless brain proteins, when prions become misfolded, they turn into contagious pathogens that recruit any other prions they come into contact with, grouping together in clumps that damage other cells and eventually cause the brain itself to break down.
If that's not bad enough, mutated prions can't easily be killed by heat or radiation, meaning once they've come into contact with something like surgical tools, they can potentially spread to other patients.
Prion-related diseases - collectively known as transmissible spongiform encephalopathies (TSE) - are fatal and untreatable, and since their discovery in the mid-80s, scientists haven't made much headway when it comes to figuring out how prions turn against us, or how to stop them.
But now researchers from Iowa State University have shown for the first time how, on the molecular level, copper ions cause prions to misfold, and then spread that mutation to other prions.
To be clear, they haven't linked those copper ions to prion-related diseases just yet - only the misfolding in the first place.
But that's a huge deal because, technically speaking, proteins shouldn't be able to infect other proteins - they're not alive, after all - and scientists have never really been able to explain the behaviour of prions - hence their reputation as the weirdest molecules ever.
The link between prions and copper was first noticed back in 2009, and now the Iowa State team has shown in molecular detail exactly how the two interact.
"Our study establishes a direct link, at the molecular level, between copper exposure and prion protein neurotoxicity," the researchers write in Science Advances.
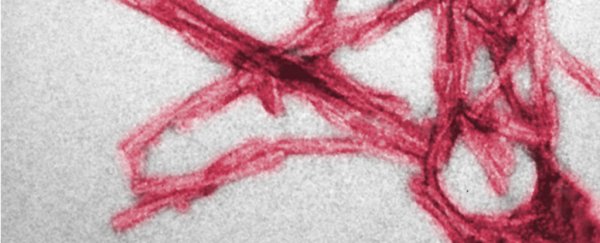
Using a super-powerful imaging technique, the researchers were able to show that misfolding begins when copper ions bind to the tail of the prion protein - and these copper ions can seed misfolding in nearby prions.
They then found that misfolded prions stick together nearly 900 times more efficiently than healthy ones.
In mice, the team also showed that these copper-induced changes were associated with inflammation and damage to nerve cells in the brain, but lead researcher Sanjeevi Sivasankar cautioned that they haven't yet investigated how infectious prion diseases spread.
"There are different strains of misfolded prion proteins and not all of them are pathogenic," said Sivasankar. "Although we do not show that the strains generated in our experiments are infectious, we do prove that copper ions trigger misfolding of prion proteins which causes toxicity in nerve cells."
The next step of their research will look into whether it's the copper-induced misfolding that is also causing diseases such as kuru and Cruetzfeldt-Jakob - or 'mad cow' disease - which could take us one step closer to working out how to stop them.
Prion-like behaviour has also been linked to Alzheimer's and Parkinson's disease, which are thought to be caused, in part at least, by amyloid beta proteins misforming and clumping together in the brain.
There's now also mounting evidence that Alzheimer's could be transmitted via surgical procedures, which makes it even more similar to a prion disease than previously thought.
"This study has major implications to our understanding the role of metals in protein misfolding diseases including prion, Alzheimer's and Parkinson's diseases," said one of the team, Anumantha Kanthasamy.
We can't wait to find out more about prions, because the more we learn, the less terrifying and more manageable they'll become. In the meantime though, still weird AF.