The Moon is our closest companion in the vast, unknowable expanse of the Universe, and yet, after centuries of hypothesising, we still can't say for sure how our little lunar satellite first came to be.
In the search for actual, empirical evidence that could clear up the mystery once and for all, researchers have made a rather unexpected discovery - the conditions created by humankind's first nuclear bomb are remarkably similar to the violent chemistry that formed our Moon.
For decades now, the leading hypothesis for the Moon's formation has been that our lunar satellite was the result of a catastrophic collision between Earth and a hypothetical, Mars-sized object called Theia.
It's the scenario most scientists can agree upon, but consensus over a really solid hunch isn't enough - in order to prove that this is how the Moon formed, we need solid evidence to take it from hypothesis to reality.
One big problem is Theia - the hypothetical planet is thought to have slammed into Earth so hard, it disintegrated into tiny pieces, and these pieces either flew off into space and formed the Moon, or mixed in with Earth's geology, and that mixture blasted into space to form our Moon.
Regardless of which scenario you think is most likely, no clear evidence of Theia's remains has been found. But if we can at least prove that the great explosion happened, we could be getting somewhere.
One of our biggest leads are volatiles - organic chemicals with really low boiling points that are thought to have evaporated from the early Moon as a result of the collision.
That assumption is based on the fact that the Moon rocks have a surprisingly low level of volatiles today - such as water and zinc - whereas Earth has an abundance.
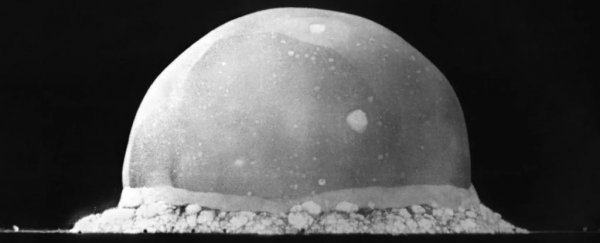
But how do scientists emulate an explosion mighty enough to prove that? Enter Trinity - the code name of the first ever detonation of a nuclear weapon.
"It dawned on me that if we're going to use a big experiment, it needed to be something of sufficient size to see [this effect]," James Day from the Scripps Institution of Oceanography at the University of California, San Diego told The Verge.
"Laboratory experiments can barely reach such high-pressure-temperature conditions."
Day and his team suspected that the remains of the Trinity test site in New Mexico - where the first plutonium bomb was detonated in 1945 - could mirror the conditions of the kind of explosion that formed the Moon.
As Leah Crane reports for New Scientist, the explosion released the energy equivalent of 20 kilotonnes of TNT, and brought the area around the bomb to temperatures over 8000°C (14,432°F) and pressures nearing 80,000 atmospheres.
"It is as close as we can probably get to conditions that you might envisage on a planetary body in the early solar system," Day told her.
The sand beneath the explosion melted into a thin sheet of greenish hued radioactive glass called trinitite, and the researchers collected samples from between 10 and 250 metres (32 to 820 feet) from ground zero.
 Trinitiite samples. Credit: Day et al. Sci. Adv. 2017
Trinitiite samples. Credit: Day et al. Sci. Adv. 2017
They then analysed these samples to see how many volatiles they'd lost in the explosion.
"Our expectation was that when we measured these trinitites, these glasses that formed in the nuclear detonation, that we were either going to see nothing … that in fact, our hypothesis that there was volatile loss during this event was wrong," Day told Eva Botkin-Kowacki at The Christian Science Monitor.
The other possibility, was that they would see the same theoretical values that have been calculated for the Giant Impact model, and Day said it was "startling" how close the zinc composition of the glass was when compared to lunar rocks brought home by the Apollo missions.
Not only did they find that the closer to the explosion the trinitite formed, the less zinc it contained, but the ratios of zinc in the trinitite were remarkably similar to those found in Moon rocks.
In fact, they found that the degrees to which the heavy and light isotopes of zinc separated from each other during the explosion were an exact match.
"The results show that evaporation at high temperatures, similar to those at the beginning of planet formation, leads to the loss of volatile elements and to enrichment in heavy isotopes in the left over materials from the event," Day says in a press statement.
"This has been conventional wisdom, but now we have experimental evidence to show it."
While the experiment does provide supporting evidence for the fact that a giant explosion was the likely cause of the Moon's formation, some big gaps remain - proof of the existence of Theia remains elusive, and critics have questioned if using analogous evidence like this is a good idea.
But the parallels the team has found between trinitite and lunar rocks are intriguing, and this big event in Earth's history could well be pointing us in the right direction to find the truth about the Moon.
"We used what was a history-changing event to scientific benefit, obtaining new and important scientific information from an event over 70 years ago that changed human history forever," says Day.
The study has been published in Science Advances.