A new system that uses sunlight to convert waste carbon dioxide into valuable chemical products - like biodegradable plastics, pharmaceuticals, and liquid fuels - has been demonstrated by scientists in the US.
In their hybrid system, metal nanowires and bacteria work together to mimic photosynthesis - the process whereby organisms can harvest energy from sunlight to produce nutrients from carbon dioxide and water.
But rather than producing nutrients, this engineered system uses sunlight to convert captured carbon dioxide emissions and water into acetate - a versatile chemical building block that can be used to synthesise more complex molecules.
While it's still some way off being commercially viable, a scaled-up version of the system could one day provide an alternative to carbon capture and storage, offering a clean option to keep carbon dioxide emissions from entering the atmosphere.
"We believe our system is a revolutionary leap forward in the field of artificial photosynthesis," said chemist and lead researcher, Peidong Yang, from the University of California Berkeley, in a press release.
"Our system has the potential to fundamentally change the chemical and oil industry in that we can produce chemicals and fuels in a totally renewable way, rather than extracting them from deep below the ground."
The system is comprised of vertically arranged silicon and titanium oxide nanowires. These wires absorb sunlight, which triggers the reduction of carbon dioxide. This structure of wires is then populated with bacteria.
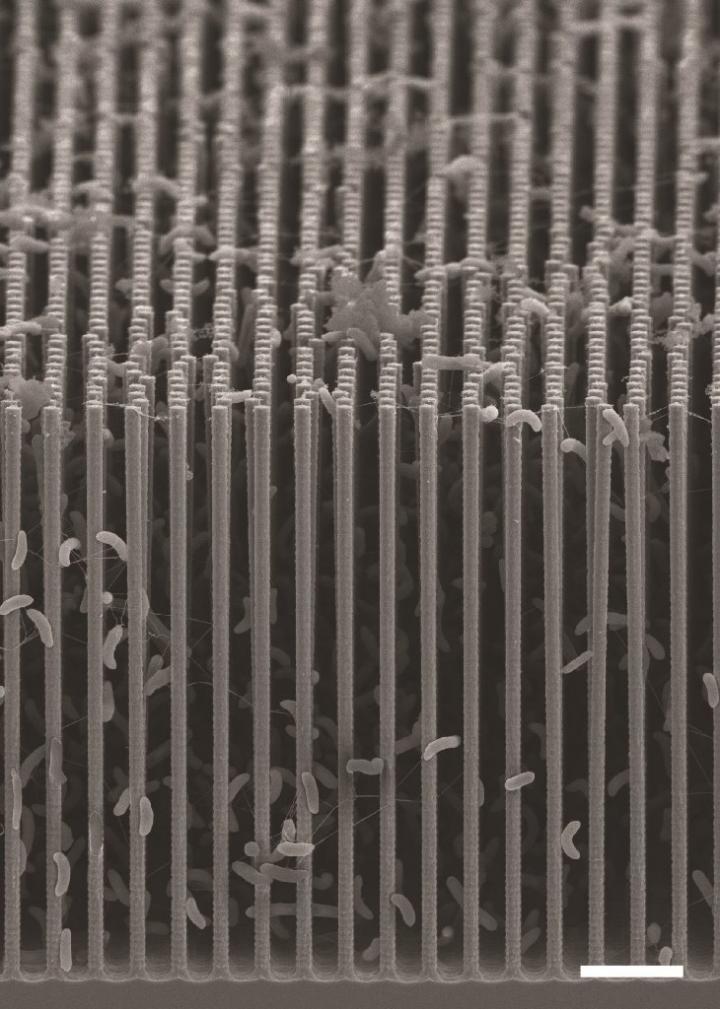 This is a cross-sectional SEM image of the nanowire-bacteria hybrid array used in a revolutionary new artificial photosynthesis system. (Credit: Berkeley Lab)
This is a cross-sectional SEM image of the nanowire-bacteria hybrid array used in a revolutionary new artificial photosynthesis system. (Credit: Berkeley Lab)
For this study, the team used Sporomusa ovata, a type of anaerobic bacteria that readily accepts electrons directly from the surrounding environment and uses them to reduce carbon dioxide.
"S. ovata is a great carbon dioxide catalyst as it makes acetate, a versatile chemical intermediate that can be used to manufacture a diverse array of useful chemicals," said co-author Michelle Chang, from UC Berkeley, in the release.
Once the carbon dioxide has been reduced by S. ovata to acetate, genetically engineered E.coli are used to synthesise targeted chemical products.
The team achieved a solar energy conversion efficiency of up to 0.38 percent for about 200 hours under simulated sunlight, which is about the same as that of a leaf, they say. But they still have some way to go before their system is solving the world's carbon dioxide storage problem.
The team says it's now working on a second-generation system, which has a solar-to-chemical conversion efficiency of three percent. They say if 10 percent efficiency can be attained in a cost-effective way, then the technology could become commercially viable.
The researchers have described their system in the journal Nano Letters, which is published by the American Chemical Society.
