We're not sure why it happened. Even 'when' is a topic of ongoing debate. But at some point, for some reason, our brains got big.
There are plenty of hypotheses as to how we got here, but to find supporting evidence we need experiments on the brains of chimpanzees and humans, which involves practical (not to mention ethical) challenges. So these scientists went and built their own specimens.
"It's a 'science fiction' experiment that couldn't have happened ten years ago," says cell biologist Arnold Kriegstein from the University of California, San Francisco.
The team constructed simple, biochemically active brains from chimpanzee and human stem-cells and used them to identify hundreds of genetic differences that could help explain their unique characteristics.
We're not talking just one or two here either. The researchers took cells from eight chimpanzees and ten humans and used them to generate a population of 56 specimens, providing unprecedented scope for precise measurements.
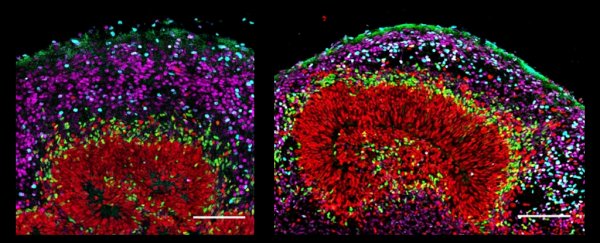
Technically the human and chimpanzee brains they developed in laboratory glassware aren't the fully developed lumps of wrinkled grey matter you'd find inside a primate's skull.
They're organoids – mixes of tissue that have self-arranged into a 3D structure to serve as a model of an organ.
While the line between an actual organ and its organoid derivative is fuzzy, it's clear these cultures of neurological tissues can't process information like the real deal. But that's not the goal.
There's sufficient genetic and biochemical activity in these cultures to allow for experiments that would be impossible on bona fide specimens.
Extracting DNA and proteins from the brains taken from deceased chimpanzees and humans and holding them side by side is like comparing the end credits of two movies. You might know the actors, but you're missing the plots.
Brain organoids allow researchers to measure how genes activate and biochemistry fluctuates, and the timing of development of important cells and other structures.
Having dozens of organoids to compare means changes that are general to each species can be picked out with precision.
The researchers deconstructed their specimens at different stages of development, allowing them to compare the types of cells emerging and the genetic programs being activated at each step.
These were all compared with reference materials taken from a third group of primates, rhesus monkeys.
Contrasts in the genetic activity of the human and chimp organoids provide fertile grounds for identifying important mutations in each species that could explain how our respective brains evolved.
"These chimpanzee organoids give us an otherwise inaccessible window to six million years of our evolution," says neurologist Alex Pollen.
"They let us ask new questions about what makes us human."
The analysis revealed 261 human-specific changes in genetic expression; one particular change that caught their interest was a type of neural precursor.
Several years ago, Kriegstein's lab identified the molecular features of a kind of cell that gives rise to the majority of human cortical neurons, called an outer radial glial cell. This time the team showed how activity in these cells ramped up their participation in a growth pathway in humans, highlighting a pivotal shift that might help explain the branching of human evolution away from our great ape relatives.
"Being so close to wild chimpanzees made me want to ask questions about our own species' evolution," says Pollen, who had studied the evolution of fish near Gombe Stream National Park's famous chimpanzee research facility.
"But first we needed genomes, stem cells, and single-cell RNA sequencing to be able to understand the evolutionary programs that drive brain development in the two species."
Whatever the story is behind the unusually enlarged brains of humans and their ilk, it's going to be complex. Organoids provide new ways to study such activity on an unprecedented scale, laying the groundwork for showing how tiny changes in our evolutionary past has led to big differences in our biology.
This research was published in Cell.