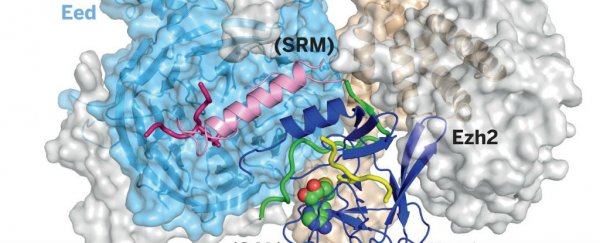
It's been a long time coming, but scientists in the US have finally figured out the atomic structure of PRC2, an enzyme complex known to play a crucial role in the development of several types of cancer.
Knowing this, they hope to understand what triggers mutations in the PRC2 gene, which has been linked to the development of lymphoma, leukaemia, and brain tumours, plus a number of congenital diseases that affect a person's growth. "Our findings bring us one step closer to understanding the chemistry of how PRC2 functions in normal cells and how mutations in the gene cause disease," said one of the team, Xin Liu, from the University of Texas Southwestern Medical Centre.
PRC2, or polycomb repressive complex 2, might not sound like much, but it plays a central role in several processes that are key to human ageing, including differentiation, maintaining cell identity and proliferation, and stem-cell plasticity.
It does this by modifying a specific protein inside chromatin - a complex of DNA and proteins that produces chromosomes inside cell nuclei - which helps it to maintain gene-expression patterns that are put in place during early development.
But when the proper functioning of PRC2 is disrupted due to mutations in the PRC2 gene, it can have very serious consequences for a person's health. "Producing either too much or too little PRC2 enzyme can unexpectedly silence or activate genes, which is not good for the cell," says Liu. "This study revealed how a 'normal' level of PRC2 enzyme activity is kept and regulated in cells."
This is exciting, because while scientists understand how PRC2 functions in general, it's interacting with hundreds, possibly thousands, of proteins in the human body at the same time, so figuring out which ones are malfunctioning and therefore triggering the development of cancer and disease has been practically impossible.
But now, for the first time, Liu and his colleagues have reproduced the 3D atomic structure of PRC2 crystals, using an imaging technique called x-ray crystallography. This means we can finally compare exactly how it behaves in normal and diseased cells, and how its interactions with chromatin and cellular proteins affect human cell growth.
The next step is to apply the findings to research that is already investigating how new drugs could inhibit PRC2 enzyme activity. According to Liu, researchers are looking at the potential of such drugs as a treatment for several types of lymphoma. "Indeed, several clinical trials are currently ongoing to target PRC2, and we believe our work will shed light on these and other studies in drug development by offering insights into how PRC2 works at the atomic level," he said.
The findings were published in the journal Science.