For the first time, scientists have created an accurate simulation of a virus invading a cell, and say it could lead to anti-viral therapies that are much more effective than the ones we rely on today.
The experiment was designed to examine how the protein shell of a virus, known as the capsid, changes as it prepares to inject genetic material into a healthy cell - changes that haven't been fully observable in previous research.
While viral infections have been simulated in the past, researchers from the Penn State College of Medicine wanted to see if they could more accurately represent the process.
Earlier simulations of viral infection showed a shift in the shape of the whole capsid, but the Penn State team suspected that this change was the result of the modelling processes - which have used extreme heat or exposed the virus to proteins.
Instead of a total change in shape, the team hypothesised that only the part of the virus that interacts with receptors on the cell should change shape during infection.
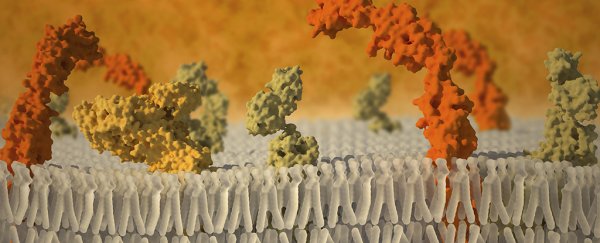
So together with colleagues from the University of Pittsburgh School of Medicine, the researchers used mock membranes called nanodiscs to create an artificial cell surface.
Into this artificial cell surface, they inserted protein molecules from human cell receptors, to allow for outside signals into the 'cell' - something that has never been done before.
An imaging technique called cryo-electron microscopy - through which beams of electrons can identify protein structures in minute detail - was then used to monitor the reaction between the virus capsids and the artificial receptor membranes.
A recent update to cryo-electron microscopy, called direct electron detection, enabled the team to capture atomic-resolution images at unprecedented speeds. Thousands of 2D images taken from different angles allowed them to finally figure out the 3D structure of the invading virus.
What they saw suggests that the researchers' hypothesis - that the virus's shape changes should only occur at the points where the cell receptors bind to the virus - was indeed correct.
"We think we have captured the first physiologically accurate virus capsid prepared to enter the host. All the ones that we had studied previously showed changes taking place all over the capsid," said lead researcher, Susan Hafenstein.
"Our work shows that a pore opens up only at that one point of interaction with the host cell And that's what's going to set up the capsid to release the genetic material into the cell."
For the purposes of the experiment, a quickly mutating virus called coxsackievirus B3 (CVB3) was chosen. Because these types of virus change as they replicate, it makes it difficult for anti-viral treatments to pin them down.
The researchers hope that by understanding more about how viruses get into cells, we can stop them more effectively. Potentially, new drugs could trigger viral mutations that prevent them from gaining access to healthy cells.
That's still some way off though. In the meantime, Hafenstein and her colleagues want to carry out more tests, and suggest that a larger nanodisc will be the next step forward.
"Because the nanodiscs in this set of experiments were so small, we're not getting the best picture of the interaction, and that's one place to improve," said Hafenstein.
Maybe then, new tests might reveal what she calls "the most important step": figuring out what triggers the release of the RNA into the cell.
Viruses, consider yourselves on notice.
The study has been published in Science Advances.
Update 1 October 2016: We've amended the copy to clarify that the team didn't make a model of the invading virus, but were able to solve its 3D structure using advanced imaging.