For the first time, scientists have successfully demonstrated a 'gene drive' in mammals, using genome editing techniques to affect the way genetic traits are inherited in a population of mice.
Gene drives have been developed before in previous animal experiments, but so far their use has only been demonstrated on insects. By showing the same concept is achievable with more complex animals, the research significantly advances the reach of an undeniably powerful (and controversial) technology.
In the theory of classical genetics called Mendelian inheritance, there's a 50/50 chance that maternal copies of any particular gene will be inherited by offspring.
At its core, a gene drive – described as a kind of Super-Mendelian inheritance in the new study – tweaks those probabilities so one type of gene (and hence trait) becomes more likely in the next generation of an organism.
"A gene drive biases the transmission of one of the two copies of a gene such that it is inherited more frequently than by random segregation," the authors of the new research, led by senior researcher and geneticist Kimberly Cooper from UC San Diego, explain in their paper.
"To our knowledge, however, such a system has not yet been demonstrated in mammals."
At least, not before now.
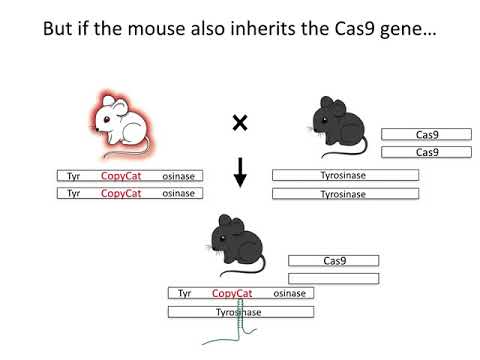
In the new research, designed as a test case, the team used CRISPR-Cas9 genome editing to maximise the chance mouse offspring would inherit a specifically engineered variant of the tyrosinase gene (Tyr, which affects mouse coat colour) from their parents.
"We've shown that we can engineer a gene that can use CRISPR and homology directed repair to 'find and replace' the non-engineered version," Cooper told ScienceAlert.
"This is the fundamental mechanism of a CRISPR-mediated gene drive, but there are many other reasons one might want to increase the rate of inheritance of a particular version of a gene."
In the insect world, for example, advocates of gene drives propose the technology could help us wipe out diseases like malaria, by altering, inhibiting, or eradicating the species of mosquito that carry the infection.
But while there are benefits, there are also potential risks, such as what unintended impacts engineered species might end up having in the wild.
"The risks that most are concerned with are the chance that genetically modified organisms might not be limited to the intended geographic region and that engineered genes could persist in the wild," Cooper says.
"All of that said, the scientists who are doing this work are well aware of these risks, and all that I know are thinking deeply about how to design gene drive systems that could be self-limiting and/or extinguished in some way."
In the new work, Cooper's team had partially mixed results, and weren't able to successfully alter the male germline without creating high rates of DNA damage.
With females, however, the techniques proved markedly more successful.
"Ordinarily one of the two versions of a gene would be passed to 50 percent of offspring," Cooper told ScienceAlert.
"We increased that to as high as 86 percent."
Because mice take significantly longer to breed than mosquitoes, it took two years to engineer results that would only have taken a few months with insects, Cooper says.
The effects might be slow, but if the technology can be perfected, gene drives in mammals could be used to fight pests or disease-spreading animals – although we have to be careful.
"Because gene drives have the potential to alter an entire species, appropriate regulation of this technology is a major concern," writes geneticist Bruce R. Conklin from the Gladstone Institute of Cardiovascular Disease in a commentary on the new research.
"Only the most intractable and major health challenges should be considered for possible interventions using gene drives."
That may be the case, but given the rate at which CRISPR technology is advancing, those interventions are becoming vastly more possible all the time.
While the bigger picture remains unknown, in Cooper's lab, the experiments continue.
"The next step is to try to improve the efficiency in females to to try to get it to work in males," she says.
"That work is ongoing, and we don't yet know the outcome."
The findings are reported in Nature.
