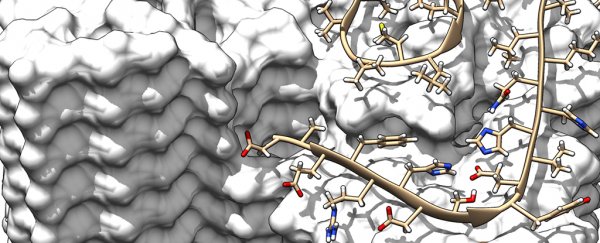
Researchers have come up with the most valuable pictures yet of amyloid fibrils that can form in the body, the solid protein aggregates associated with diseases such as Alzheimer's.
Thanks to the new high-resolution, atomic-level scans, scientists are hoping to be able to better understand how Alzheimer's takes root in the brain, and what can be done to stop these fibrils from spreading.
This latest model shows the intricate interactions and exact positions of the amyloid beta (Aβ) proteins as they form fibrils, something we haven't been able to get such a close look at before, say the researchers from Germany and the Netherlands.
"This is a milestone on the road to a fundamental understanding of amyloid structures and the related diseases," says one of the team, Dieter Willbold from the Heinrich Heine University Düsseldorf in Germany.
"The fibril structure answers many questions about the mechanism of fibril growth and identifies the role played by a whole series of familial mutations that lead to early onset of Alzheimer's disease."
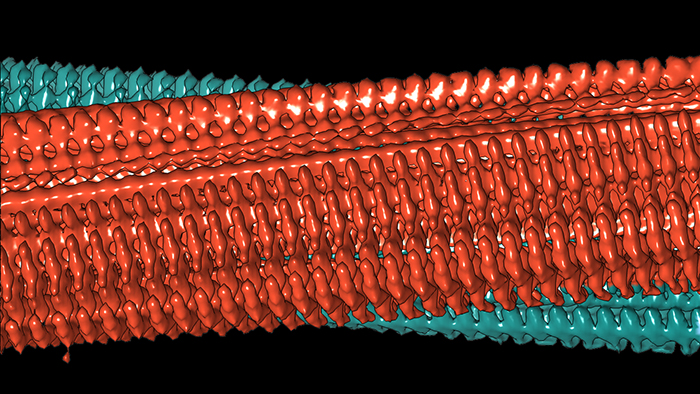 Diagram of two protofilaments forming a fibril. Credit: Forschungszentrum Jülich / HHU Düsseldorf / Gunnar Schröder
Diagram of two protofilaments forming a fibril. Credit: Forschungszentrum Jülich / HHU Düsseldorf / Gunnar Schröder
Amyloid fibrils are made up from naturally occurring proteins that are normally soluble, but which can go astray and mesh together to form insoluble fibres.
In the case of Alzheimer's, these fibril plaques build up between nerve cells and are the prime suspects for causing the cell death and tissue loss associated with the disease.
To get a detailed look at these fibrils, the researchers made use of a cryo-electron microscopy facility, which uses incredibly low (cryogenic) temperatures to keep atoms in place and measure them without interference in their natural state.
Extra readings from solid-state nuclear magnetic resonance (NMR) spectroscopy and X-ray diffraction experiments helped to validate the data and the atomic structure, producing a computer rendering that's the most accurate we've ever seen, down to a resolution of 4 angstroms or 0.4 nanometres.
"The individual images in cryo-electron microscopy are usually extremely noisy since proteins are very sensitive to electron radiation and the pictures can only be generated with very low radiation intensity," explains one of the researchers, Gunnar Schröder, also from the Heinrich Heine University Düsseldorf.
The breakthrough came by scanning and analysing multiple fibrils that shared much of the same shape and symmetry, which the team achieved by restricting their growth. That meant thousands of similar pictures could be combined to make one 3D scan.
And it turned up some useful findings, like the way single Aβ protein molecules are staggered in layers on top of each other. Two of these so-called protofilaments can combine to form a fibril, and several entangled fibrils then form plaques.
In other words the individual molecules in these entangled protofilaments are staggered, like a zipper, locking them in place.
The new high-resolution scans also show the location and structure of all the 42 amino acid residues from individual Aβ protein molecules. That in turn could help explain how genetic variations affect the chance of developing Alzheimer's – different Aβ protein variants apparently change the stability of the fibrils.
We know that mice don't get Alzheimer's and we know that a small section of the population in Iceland seem to be immune to it, and the new imagery can give scientists clues as to the genetic reasons why.
There's still a lot we don't understand about Alzheimer's, but we're making progress, and the new amyloid fibril imagery should help inform the study of Alzheimer's and related diseases for years to come.
The research has been published in Science.