An international team has managed to capture an elusive state of matter that stays stable even at room temperature. It's called a supercrystal, and all it took were laser pulses shorter than a blink of the eye.
Okay, so of course it's more complicated than that. The experiment involved layering inorganic compounds and "frustrating" them, which meant building a structure that prevents the materials from naturally achieving their 'preferred' state of crystallisation.
As the frustrated materials were hovering in a disorganised manner, a special laser technique helped the team to suspend the materials in a highly ordered state - the supercrystal.
"We are looking for hidden states of matter by taking the matter out of its comfortable state, which we call the ground state," said materials scientist Venkatraman Gopalan of Penn State.
"We do this by exciting the electrons into a higher state using a photon, and then watching as the material falls back to its normal state. The idea is that in the excited state, or in a state it passes through for the blink of an eye on the way to the ground state, we will find properties that we would desire to have, such as new forms of polar, magnetic and electronic states."
You can't make supercrystals out of any old matter. The team used alternating layers of single-atom thick lead titanate and strontium titanate, stacked into a three-dimensional structure. They grew these layers on a base (substrate) of dysprosium scandium oxide, whose crystals are in between the size of crystals formed by the two other materials.
This unique build let the researchers achieve the frustration we mentioned earlier.
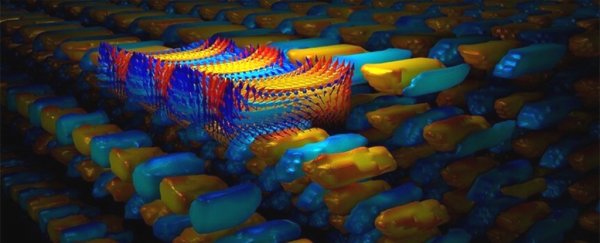
Lead titanate is ferroelectric, a material that has positive and negative electrical poles. Strontium titanate is not ferroelectric, and as these materials were layered, the electric polarisation vectors had to contort into weird pathways, curving back on themselves to create vortices.
The size of crystals in the base provided the last necessity: the strontium titanate tried to stretch to match the size of the substrate's crystals, and the lead titanate tried to compress. The result is a peculiarly disorganised, frustrated system, with multiple states distributed throughout the material.
The team then used what they call a "pump-probe" laser technique. A femtosecond pulse of blue laser "pump" light is flashed, lightning-like, onto the structure, which excites the electrons. This is followed by the "probe" light, a more gentle pulse that reads the state of the matter.
They found that, rather than slipping back to its disordered state, as might be expected, the matter was trapped in an intermediate supercrystal state indefinitely - even at room temperature, unless heated above 176 degrees Celsius (350 degrees Fahrenheit).
"By virtue of its short pulse duration, an ultrafast laser imprints excitations in materials faster than their intrinsic response time," said materials scientist Vlad Stoica of Penn State and Argonne National Laboratory.
"While such dynamical transformations were already explored for decades to stimulate the ordering of materials, a strategy for their steady state stabilisation seemed out of reach until now."

A supercrystal typically has abnormally large unit cells - the smallest repeating unit in a crystal's three-dimensional structure.
The supercrystal achieved in this study had unit cells with a volume at least a million times greater than the unit cells of the lead titanate and strontium titanate, all organised like soldiers in formation.
In room temperature, this formation remained stable for at least a year, and potentially could stay stable indefinitely.
"For the first time, we observed that a single ultrafast laser pulse irradiation of artificially layered polar material can induce long-range structural perfection when starting from relative disorder," said the research team at Argonne National Laboratory.
"This experimental demonstration has already stimulated theoretical developments and has important implications toward future realisation of artificial nanomaterials that are not achievable by traditional fabrication."
The research has been published in Nature Materials.