Self-taught venom expert Tim Friede has voluntarily injected himself with snake venom 856 times across 18 years. Now, in spite of the odds, Friede's extremely dangerous hobby has led scientists to create the most widely effective snake antivenom on record.
Collecting pet snakes since his youth, Friede first began to deliberately envenom himself by milking his pets, diluting their venom, and injecting it repeatedly. When he received two separate cobra bites in the space of an hour, the venom almost killed him.
"I basically flat-lined and died," he told National Geographic's Dominic Bliss. "It wasn't fun. I had enough immunity for one bite, but not for two. I completely screwed up."
Many of us might take such a near-death experience as a sign to find a new pastime, but Friede saw it differently. His self-envenomation regime, he realized, was probably the main reason he survived the incident. He doubled down.
In the following years, Friede's immune system faced horrors unknown. Either by syringe or by fang, his B cells – white blood cells that create the pathogen-fighting antibodies that protect us from foreign substances like venom, viruses, bacteria and parasites – were introduced to the toxic bites of Egyptian cobras, water cobras, coastal taipans, Mojave rattlesnakes, even black mambas.
And, like any young man doing seemingly foolish things in the early 2010s, he recorded it all on his phone and uploaded it to YouTube. Don't watch this unless you want to see a man bitten by a Papua New Guinea taipan and a black mamba in quick succession.

This personal project could have easily earned Friede a Darwin award, or in the very least a guest appearance on Jackass. But luckily, his cells began to produce antibodies that protected him from the toxic regimen.
Friede is still alive, and his unique antibodies are actually being put to good use. His YouTube videos attracted the attention of Jacob Glanville, immunologist and CEO of biotech company Centivax.
Using Friede's hyperimmune antibodies, Glanville and a team of scientists have now created an antivenom that, in lab experiments, protected mice from the venom of 19 different snake species, all listed by the World Health Organization as category 1 and 2 of the world's deadliest snakes.
Usually, antivenom is created by collecting the antibodies produced by sheep or horses that have been repeatedly exposed to venom from just one snake species each. That's why antivenoms tend to be specific to a species and region, which, as any outdoor enthusiasts will know, makes it difficult to pack a comprehensive first aid kit. And because the antibodies aren't from humans, there's always a risk of adverse reactions.
An antivenom derived from Friede's blood, on the other hand, could protect against a range of species with fewer complications.
The antivenom tested by the team is composed of two different antibodies isolated from Friede. The first, LNX-D09, was effective against six of the snake species tested on mice. When paired with a drug called varespladib, the antivenom barrier shielded mice from the venom of three more species of snake.
The second type of Friede's antibodies, SNX-B03, extended at least partial protection to the entire panel of species' venoms.
"By the time we reached three components, we had a dramatically unparalleled breadth of full protection for 13 of the 19 species and then partial protection for the remaining that we looked at," says Glanville. "We were looking down at our list and thought, 'what's that fourth agent'? And if we could neutralize that, do we get further protection?"
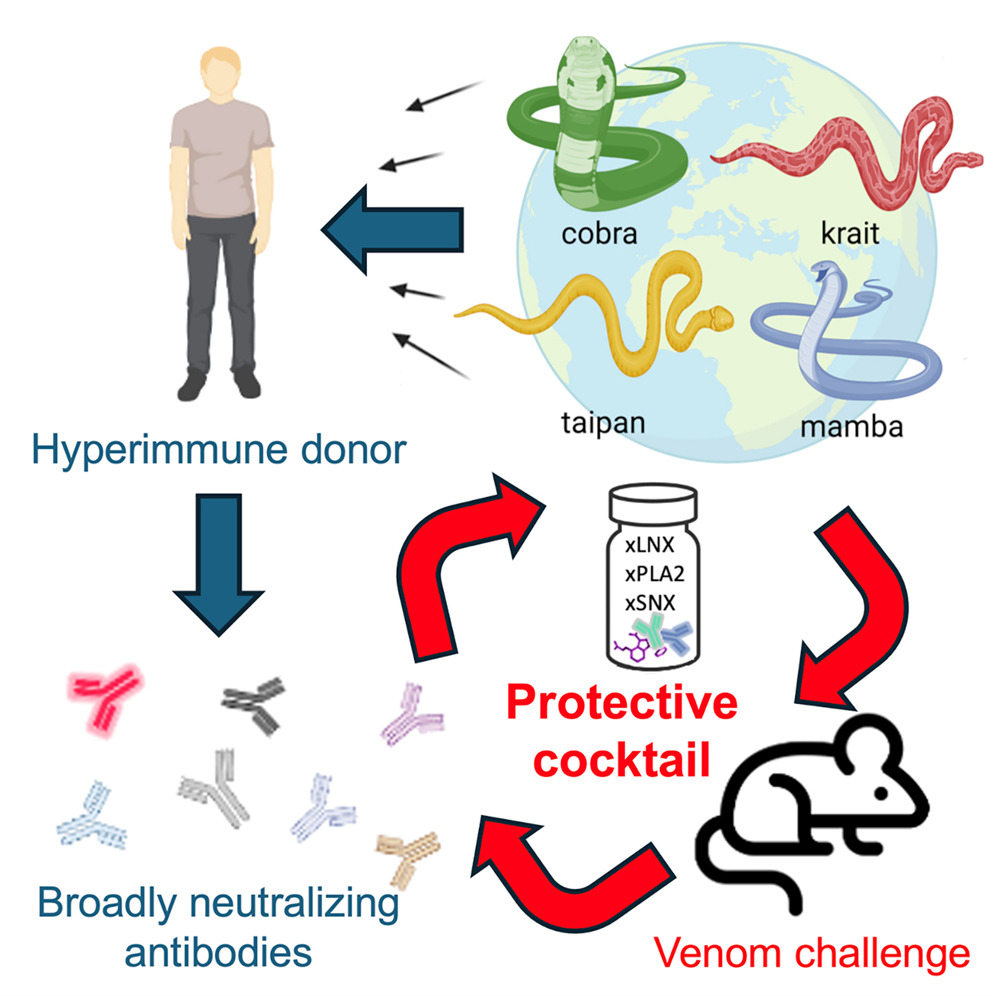
Glanville has universal antivenom in mind: a single cocktail that could save anyone, anywhere, from any species of snake that might have bitten them. What his team has developed so far brings this closer to being realized.
This work focused on one major family of venomous snakes, known as the elapids, and it may work against other species in that family that weren't directly tested. In time the team hopes to develop a similarly wide-acting antivenom for the other main family, the viperids.
"We're turning the crank now, setting up reagents to go through this iterative process of saying what's the minimum sufficient cocktail to provide broad protection against venom from the viperids," says biologist Peter Kwong at Columbia University.
Rigorous clinical testing will be needed before the antivenom could become available to humans. In the meantime, the researchers plan to conduct field trials of the antivenom to treat snake-bitten dogs presented to veterinary clinics in Australia.
The research was published in Cell Press.
