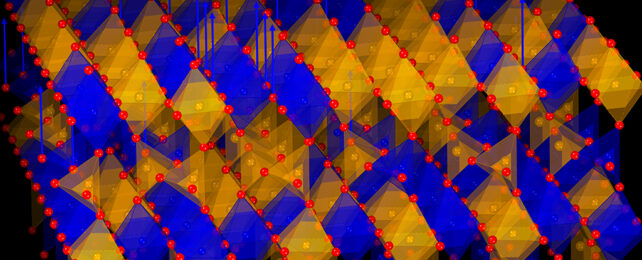
Scientists have discovered a metal oxide with the catchy name SrFe0.5Co0.5O2.5 (or SFCO for short) that can 'breathe', taking in oxygen atoms and releasing them again at relatively low temperatures without breaking.
What makes that so important is that these materials, known as transition metal oxides, can essentially be reprogrammed with oxygen atoms. As atoms are added or removed, the material's properties – including magnetism and conductivity – can be changed.
This proof-of-concept potentially provides a new degree of control over behavior of materials commonly used in electronics, clean energy systems, and buildings, via oxygen in the surrounding gas.
Related: Spiral Magnetism Seen in Synthetic Crystal For The First Time
SFCO is made up of strontium, iron, and cobalt, but only the cobalt atoms were changed by the 'breathing'. That points to opportunities to fine-tune materials more precisely in the future.
"This finding is striking in two ways," says physicist Hyoungjeen Jeen, from Pusan National University in South Korea.
"Only cobalt ions are reduced, and the process leads to the formation of an entirely new but stable crystal structure."
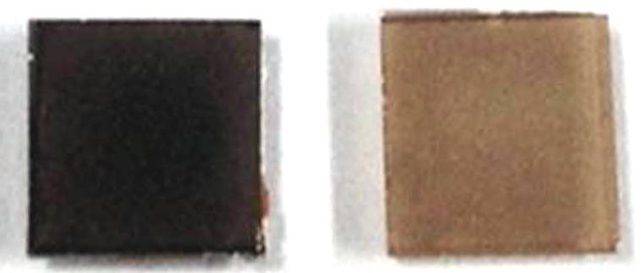
As oxygen was removed from wafer-thin sheets of the material, it became more transparent and more insulating (so electrical resistance increased). The crystal structure also became slightly larger.
Another important discovery noted by the researchers is that the process is reversible: not only can oxygen be removed, but SFCO goes back to normal when the oxygen returns. That's another useful property for material engineering.
While the researchers suspected SFCO would offer up some interesting shifts in state, they weren't expecting the complete rearrangements caused by the addition and subtraction of oxygen.
"It is like giving the crystal lungs and it can inhale and exhale oxygen on command," says Jeen.
One potential application is in solid oxide fuel cells, producing electricity from hydrogen. These cells rely on the process demonstrated here: taking up and releasing oxygen in a way that's stable, reversible, and somewhat practical.
Speaking of practicality, while the researchers are keen to talk up the relatively normal conditions that their experiments can work in, we're still talking about very specific lab environments without any external interference.
That's something that can be worked on in future studies, but this represents a significant advance for scientists looking to find materials that can be precisely programmed and switch between different states without any damage – and we can expect to hear about other breakthroughs based on the research done here.
"This is a major step towards the realization of smart materials that can adjust themselves in real time," says chemist Hiromichi Ohta, from Hokkaido University in Japan.
The research has been published in Nature Communications.