Mutations in a gene associated with Crohn's disease have been found to rob critical immune cells of their ability to switch modes, causing them to overreact and trigger inflammation.
Variations in the NOD2 gene have been linked to Crohn's in previous studies, yet their exact role in the disease's pathology has long been a mystery.
Researchers from the University of California, San Diego used machine learning techniques to identify patterns in gene activity of immune cells in the gut.
Experiments on lab-grown cells and samples from both healthy guts and digestive tracts with a form of Crohn's called inflammatory bowel disease (IBD) revealed that the mutations interfere with typical protective mechanisms that allow NOD2 proteins to guard against IBD.
Related: These DNA-Damaging Molecules May Be The Link Between Colon Cancer And IBD
Tracking the behavior of immune cells called macrophages through the expression of their genes, the researchers discerned which cells helped the gut stay healthy and which became inflammatory and caused damage.
"The gut is a battlefield, and macrophages are the peacekeepers," says UC San Diego Gajanan Katkar. "For the first time, AI has allowed us to clearly define and track the players on two opposing teams."
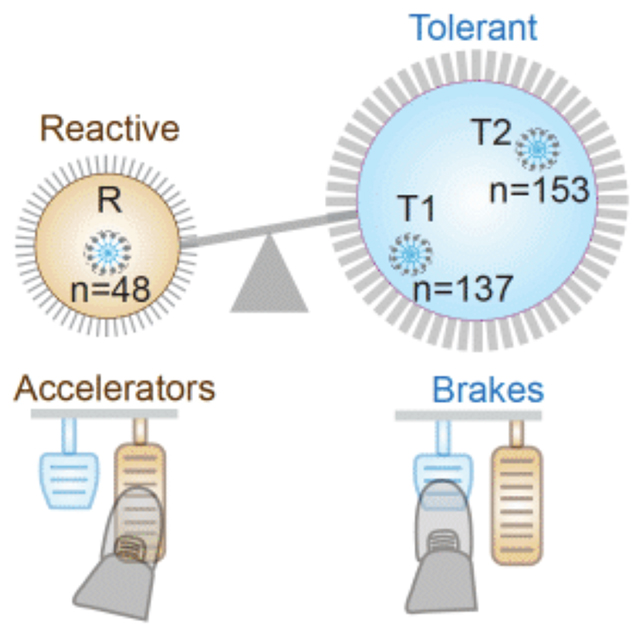
Macrophages in the gut can switch between two states, one for attacking infection (inflammatory) and one for repairing damage (non-inflammatory). It's been well established that keeping these two states balanced is important for a healthy gut.
The research team put together a genetic signature involving 53 genes, which controlled the macrophage state in IBD.
One gene found to promote the non-inflammatory macrophage mode produces a protein called girdin. It turns out that the girdin and NOD2 proteins work together to ensure macrophages remain alert to threats, but not overly reactive. Without them, macrophages in repair mode are less efficient at cleaning up targets, while those in attack mode are overly inflammatory.
"NOD2 functions as the body's infection surveillance system," says cell biologist Pradipta Ghosh, also of UC San Diego. "When bound to girdin, it detects invading pathogens and maintains gut immune balance by swiftly neutralizing them."
"Without this partnership, the NOD2 surveillance system collapses."
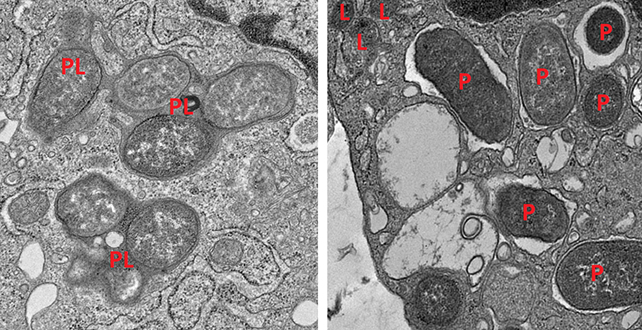
Further experiments on mice backed up the findings: Mice lacking girdin developed inflammation in their guts and often died of sepsis, which is caused by an overreaction of the immune system.
This gives us a much better understanding of the imbalance that helps to drive IBD and the role that NOD2 plays in Crohn's disease in particular. Crohn's is thought to be caused by several overlapping factors, but this seems to be a big part of the overall picture.
As always with this type of research, identifying the genes responsible for certain conditions gives scientists more opportunities to develop targeted treatments – so one day in the future we may have drugs that are able to keep the gut's macrophage population as well balanced as NOD2 and girdin do.
"These insights shed new light on the molecular pathways underlying gut homeostasis and the progression of IBD, offering potential therapeutic avenues for restoring balance in macrophage subpopulations," the researchers conclude in their published paper.
The research has been published in the Journal of Clinical Investigation.

