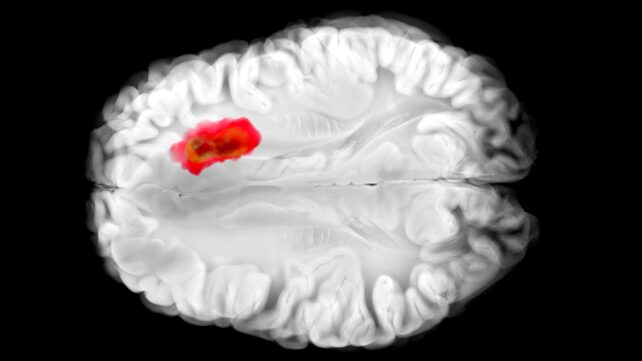
Hydralazine has been used to treat high blood pressure for many decades, even though it's never been exactly clear how it works. Now a new study answers some key questions about the medication – and adds an intriguing new link to brain cancer.
Taking a closer look at the effects of hydralazine on human and mouse cells, researchers led by a team from the University of Pennsylvania found that it blocks a particular enzyme called 2-aminoethanethiol dioxygenase (ADO).
That same enzyme, as it happens, is thought to play a role in aggressive glioblastoma brain cancers. This new understanding of hydralazine could lead the way to new cancer treatments, as well as improve the drug's effectiveness for its current targets.
Related: Serotonin Could Play an Unexpected Role in Cancer, Scientists Discover
"Hydralazine is one of the earliest vasodilators ever developed, and it's still a first-line treatment for preeclampsia – a hypertensive disorder that accounts for 5-15 percent of maternal deaths worldwide," says physician-scientist Kyosuke Shishikura, from the University of Pennsylvania.
"It came from a 'pre-target' era of drug discovery, when researchers relied on what they saw in patients first and only later tried to explain the biology behind it."
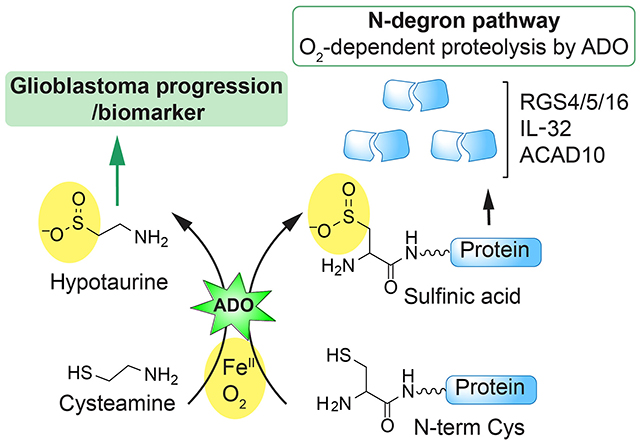
The researchers describe ADO as an "alarm bell" that alerts the body to dropping oxygen levels. It triggers a chain reaction, tightening blood vessels by destroying so-called RGS proteins (short for regulators of G-protein signaling).
Previous studies have shown that glioblastoma tumors are often high in ADO, hijacking it to produce a chemical called hypotaurine, which helps the cancer cells spread, survive for longer, and tolerate stress.
But no ADO inhibitors were known prior to this study.
Hydralazine effectively mutes ADO, the team found: RGS proteins aren't attacked, blood vessels aren't squeezed, and blood pressure drops. In experiments with human glioblastoma cells, hydralazine halted tumor growth by blocking ADO.
It's very early days – the effects of hydralzine still need to be tested in people with glioblastoma in clinical trials – but these are promising findings that could unlock a way to control the spread of these notoriously hard-to-treat brain tumors.
The newly uncovered mechanism also explains why hydralazine is an effective treatment for preeclampsia, a high blood pressure condition in pregnant women. That means the drug can be better engineered and personalized, to reduce side effects and improve results.
"Understanding how hydralazine works at the molecular level offers a path toward safer, more selective treatments for pregnancy-related hypertension – potentially improving outcomes for patients who are at greatest risk," says chemist Megan Matthews, from the University of Pennsylvania.
The discoveries here mean better drugs for both high blood pressure and brain cancer can now be developed, carefully balancing the need to hit particular pathways in cells while minimizing harm to healthy parts of the body.
And since hydralazine is already widely used, understanding its mechanism of action gives scientists something of a head start if they want to build more treatments based on the compound.
Further down the line, we might be able to take out one of the key defenses of glioblastoma, adding to the treatments already in development.
"It's rare that an old cardiovascular drug ends up teaching us something new about the brain," says Matthews, "but that's exactly what we're hoping to find more of – unusual links that could spell new solutions."
The research was published in Science Advances.