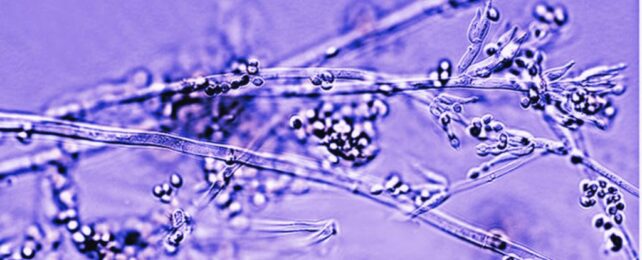
Fungi are best known for returning dead, organic matter to the Earth, but materials scientists are exploring whether they could someday help our bodies repair, in the form of special hydrogels.
To play a role in biomedical settings, a hydrogel needs a multilayered structure like our own skin, cartilage and muscles. While some engineers are working on synthetic versions that mimic biology, University of Utah scientists have found a hydrogel that literally has a life of its own.
Marquandomyces marquandii is a common species of soil mold, and a promising candidate for the job. This fungus has had a bit of an identity crisis, being misclassified as Paecilomyces marquandii until it was reassigned to its own genus in 2020. Soon, it may be able to add the role of 'bio-integrated hydrogel' to its resume.
Bio-integrated hydrogels are created from organisms that we know form crosslinked, intricate network structures that may be capable of standing in for our own soft tissues.
"Hydrogels are regarded as a promising alternative for applications in tissue regeneration and engineering, cell culture scaffolds, cell bioreactors, and wearable devices, owing to their ability to closely mimic the viscoelastic properties of soft tissues," writes lead author Atul Agrawal, an engineer at the University of Utah, and his collaborators.
While we're most familiar with fungi's mushrooms and moldy fuzz, these are actually the organism's reproductive parts. Fungi comprise mainly of a network of filaments called mycelium, usually hidden deep within the soil, wood, or that old avocado at the bottom of your fruit bowl. It's this fibrous, layered network that has made fungi such an exciting biomaterial to explore.
"As they grow forward, they lay down these cross walls that then compartmentalize a really long filament into many, many individual cells," says mycologist Bryn Dentinger from the Natural History Museum of Utah. "They will grow forever as long as there's enough nutrition around… there's a lot that we could exploit from those behaviors that really haven't been explored fully."
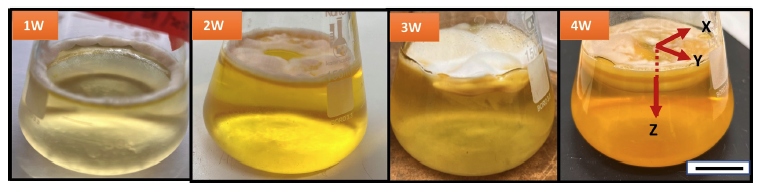
Other fungi have been tested for their potential as biomedical hydrogels, but they have often proved too brittle or dry out too fast. That was not the case for M. marquandii, which, when grown using a stationary liquid fermentation method, formed a hydrogel capable of retaining up to 83 percent water.
"What you are seeing here is a hydrogel with multilayers," says Agrawal, referring to one of the fungal colonies he is growing in a glass flask, full of the yellowish liquid culture medium.

"It's visible to the naked eye, and these multiple layers have different porosity. So the top layer has about 40 percent porosity, and then there are alternating bands of 90 percent porosity and 70 percent porosity."
They suspect these different layers arise from changes in growth rate and strategy: For instance, where the fungi reached the surface of the growth medium, its porosity was lowest, perhaps because it was prioritizing sideways growth. This means scientists could tweak the growing conditions, such as oxygen supply and temperature, to optimize the hydrogel's microstructure, depending on what it might be used for.
"This one in particular was able to grow these big, beefy mycelial layers, which is what we are interested in," says University of Utah materials engineer Steven Naleway. "Mycelium is made primarily out of chitin, which is similar to what's in seashells and insect exoskeletons. It's biocompatible, but also it's this highly spongy tissue."
"In theory, you could use it as a template for biomedical applications or you could try to mineralize it and create a bone scaffolding," Naleway adds.
It will be a long time before your doctor might offer mushroom skin as a burn treatment, or a fungi-grown hip replacement. M. marquandii is not known to be harmful to humans, but animal studies suggest the chitin in mold might trigger rare allergic reactions.
On the flip side, M. marquandii can help some plants grow. So many more experiments will be needed to see just how well this soil fungi can get along with living tissue – we can't risk a scenario like The Last of Us.
"To the best of our knowledge, this is the first report of a mycelium species achieving such hydrogel-like properties under submerged growth conditions, positioning M. marquandii as a novel and promising material for biomedical applications," the team writes.
This research was published in The Journal of The Minerals, Metals & Materials Society.