Scientists have accidentally tied the smallest and tightest knot ever, overtaking the top spot in the Guinness Book of World Records.
The remarkable microscopic tangle contains just 54 atoms that twist around three times into an interlacing loop called a 'trefoil' knot, with no loose ends.
This 'three-leaf clover' shape is the simplest of nontrivial knots, and it's fundamental to mathematical knot theory.
In 2020, chemists in China trained a chain of 69 atoms to cross over itself three times to form the trefoil. Now, researchers at the University of Western Ontario in Canada and the Chinese Academy of Sciences have joined forces and beaten that record.
As the ratio of atoms to 'back crossings' diminishes, the strength of the molecular knot grows stronger. The knot created in 2020 has a backbone crossing ratio (BCR) of 23.
The current knot scores a BCR of 18.
Most organic molecular knots have a BCR of between 27 and 33. While experts aren't sure how small or tight they can continue to make single-stranded knots, quantum chemical calculations suggest that the most stable trefoil structure is about 50 molecules long, which means we are inching closer to the theoretical limit.
The recent feat gets experts closer than ever to the microscopic knots that form naturally in DNA, RNA, and various proteins in our bodies. What's more, understanding how the newest knot forms could help scientists build better plastics and polymers.
"Molecular knots, whose synthesis presents many challenges, can play important roles in protein structure and function as well as in useful molecular materials, whose properties depend on the size of the knotted structure," explain the team of researchers.
Like many scientific breakthroughs, this one was a happy accident. The chemist Richard Puddephatt told Alex Wilkins at New Scientist how the serendipitous event happened.
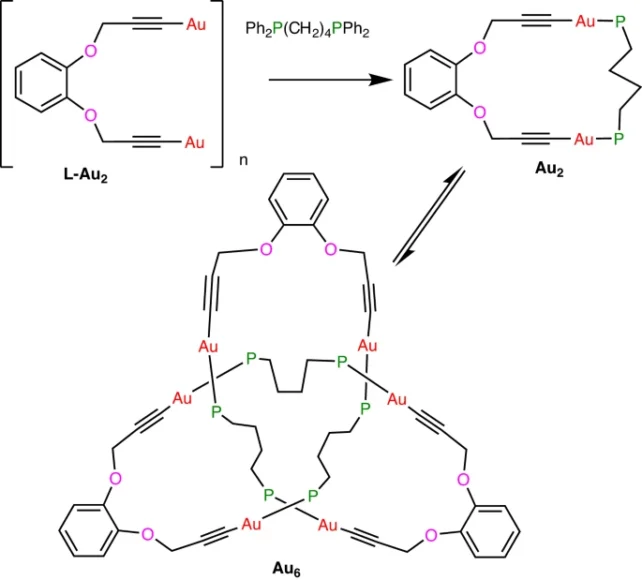
Puddephatt and his colleagues were working on creating metal acetylides in the lab, which are alkynes – a type of hydrocarbon – with hydrogen removed from the end. This end product is highly useful as it can help scientists carry out organic chemical reactions.
When connecting gold acetylide with another carbon structure called a diphosphine ligand, the team unexpectedly created a trefoil knot instead of a gold chain, or catenane.
Since 1989, chemists have been trying to tie molecular knots by guiding helical chains into a desired structure with metal ions.
In 2020, for instance, chemists tied the tightest trefoil knot of the time by using metal atoms to "fold and entwine" the molecular strand. When these metal atoms are removed at the end of the process, the knot cannot be untied.
The newest 'metallaknot' of gold is different because it assembles itself.
"It's quite a complicated system and, honestly, we don't know how it happens," Puddephatt, who works at the University of Western Ontario, told New Scientist.
He and his colleagues hope that their knot will "provide a strong motivation to pursue similar, but hopefully more robust, structures by self-assembly" in the future.
The study was published in Nature Communications.
