COVID-19 could lead to a protein build-up similar to that seen in Alzheimer's patients – not just in the brain, but also in the eyes, according to a new study.
This may explain why 'brain fog', an umbrella term for issues with memory or cognition, is often reported as a symptom of COVID-19.
In a new study led by Yale University, researchers sought to illuminate apparent similarities between COVID brain fog and Alzheimer's, investigating whether SARS-CoV-2 can lead to Alzheimer's-like plaques that might explain post-infection brain fog.
Related: Severe COVID Looks Scarily Like Old Age in The Human Brain, Study Finds
"There is growing evidence linking COVID-19 and brain fog, a commonly reported symptom following infection," says senior author Brian Hafler, ophthalmologist at Yale School of Medicine.
"While the mechanisms of brain fog after COVID-19 are not fully understood, scientists have found that SARS-CoV-2 can induce amyloid beta accumulation in the central nervous system."
Retinas are part of the central nervous system, and as two of the system's most clinically accessible parts, they may offer a window into more than just the soul.
Previous research has shown amyloid beta accumulates in the retinas as well as brains of Alzheimer's patients, suggesting retinal testing may offer a practical method for large-scale Alzheimer's diagnosis and monitoring.
To investigate, the researchers used postmortem human retinal tissue and grew retinal organoids – small, 3D retina models derived from human stem cells.
They analyzed various cell types found in retinal tissue, measuring RNA within cell nuclei to determine the protein production of different cells.
They focused on two proteins – neuropilin-1 (NRP1) and angiotensin-converting enzyme 2 (ACE2) – previously identified as possible targets exploited by SARS-CoV-2 to infiltrate neurons.
NRP1 turned up in neurons and glial cells from retinal tissue of people who had COVID, pointing to a possible entry point for viruses into human eyes.
People with no history of dementia still showed elevated amyloid beta buildup if they had a COVID history, often yielding Alzheimer's-like retinal tissue.
Amyloid beta levels also rose in retinal organoids after exposure to SARS-CoV-2's spike protein, which helps the virus enter host cells.
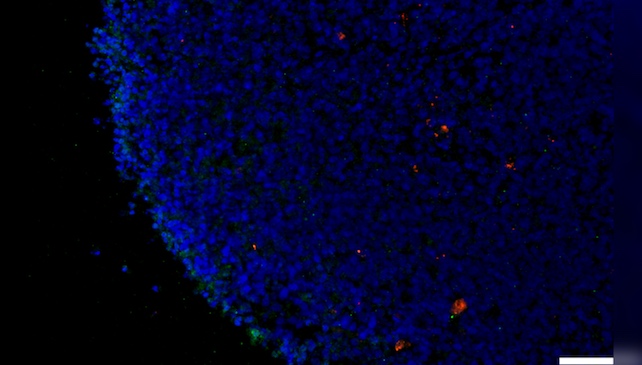
When researchers added an NRP1 inhibitor to the mix, however, they managed to counter the amyloid beta increase that otherwise occurred in retinal tissue exposed to the coronavirus spike protein.
This hints at the possibility of targeting NRP1 to combat neurological complications of COVID, such as those commonly labeled brain fog.
"Mechanistically, the involvement of NRP1 in amyloid beta aggregation gives a specific molecular target for future investigation," Hafler says.
"Our study showed that exposure to SARS-CoV-2, in particular spike protein, can lead to the formation of amyloid beta aggregates in both human retinal tissue and retinal organoids."
Beyond shedding light on COVID brain fog, the study also lends support to the idea that amyloid beta is like a bodyguard for the brain, Hafler says.
Long suspected of causing Alzheimer's, these plaques are now widely seen more as indicators of underlying danger.
Amyloid beta is still poorly understood, but it's structurally similar to known antimicrobial peptides, and some research suggests it may play an important role in the brain's immune system.
Since Alzheimer's can weaken the blood-brain barrier, amyloid beta buildup could indicate the brain's attempts to fend off microbial intruders.
"It bolsters the amyloid beta antimicrobial hypothesis of Alzheimer's disease, suggesting that amyloid beta could act as part of the brain's innate immune response against viral infections," he says.
Other viruses may trigger similar amyloid beta buildups, the authors point out, noting the need for more research to investigate.
Hafler and his colleagues, for their part, are now conducting clinical studies in hopes of revealing whether COVID can raise long-term Alzheimer's risk.
"Our ultimate goal is to prevent long-term neurological effects of COVID-19 and explore NRP1 inhibitors and other modulators of virus-host interactions as potential therapeutics for preventing viral-induced amyloid pathology and Alzheimer's disease," Hafler says.
The study was published in Science Advances.