First there's weakness and throbbing in the arm muscles that take on the immune-teaching chemicals. Then the fatigue and aches spread until your whole body is complaining.
It turns out those of us who experience this reaction to the COVID-19 vaccines have markers in our blood that indicate a more active immune response, but a new mouse study has hinted at a simple way to reduce this reaction, while still maintaining the vaccine's vital impact.
And it could be as simple as changing the inoculation technique.
Researchers from the Duke-NUS Medical School in Singapore analyzed blood samples from 175 healthcare workers who received the Pfizer vaccine. They found those who experienced fatigue expressed more genes related to the activity of T and natural killer immune cells.
"This study provides a first insight into the molecular basis of a side effect that many have experienced following mRNA vaccination," says virologist Eng Eong Ooi.
Most vaccines are administered into the muscle layer of our tissues. This is because many immune cells that learn to recognize foreign invaders reside here and muscle tissue has an excellent blood supply for easy transport elsewhere in the body.
The immune cells here can quickly take up the antigens in the vaccine and deliver them to the nearest lymph nodes; in the case of the inoculation site on your shoulder, the nearest lymph nodes are in your armpit.
In contrast, the fat layer beneath your skin has far less blood supply and immune cells, meaning your immune cells will not encounter the antigens released here or get them to the lymph nodes as fast.
This provides time for other molecules in the surrounding area to react to the antigens, possibly degrading them so that they no longer accurately represent the threat they're supposed to be teaching our immune system to be wary of.
For some vaccines, like hepatitis B and rabies, this can lead to vaccine failure.
But researchers compared two inoculation sites for the Pfizer COVID-19 vaccine in mice: muscle and subcutaneous where the vaccine is placed in the fat layer, above the muscle layer. They found that subcutaneous inoculations were just as effective.
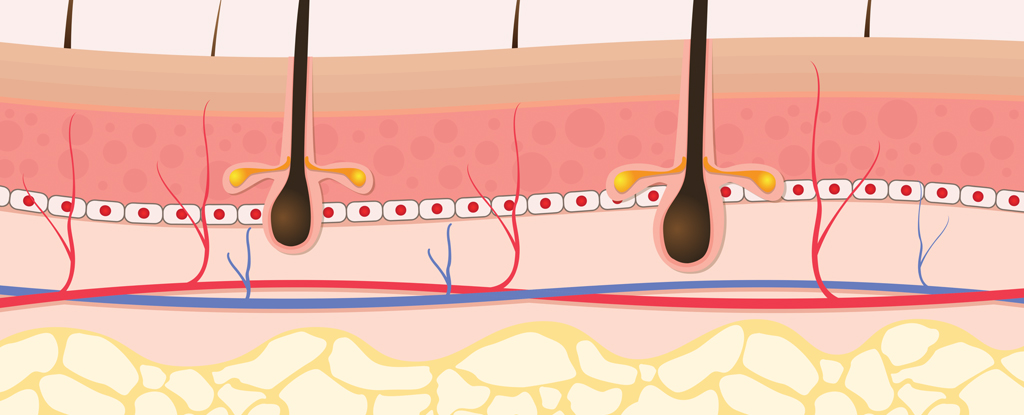 Diagram of skin layers with lowest depicted being the subcutaneous layer. (chuteye/Getty Images)
Diagram of skin layers with lowest depicted being the subcutaneous layer. (chuteye/Getty Images)
What's more, the mice who received a subcutaneous inoculation experienced less expression of genetic markers for inflammation – both locally and across the mice's whole system.
While further research will of course be needed to ensure the response is the same in humans, this study provides a solid understanding of the underlying mechanisms involved. It also revealed some unexpected results.
The researchers were surprised that mice's immune responses to the subcutaneous inoculations were stronger than the muscle ones. While it's still unclear why, they suspect the additional inflammation experienced after a muscle inoculation may actually suppress some immune cell responses – specifically killer T-cells.
"This suggestion is consistent with the finding that in severe COVID-19 patients, where hyperinflammation is a feature, T-cell response is muted compared to those with uncomplicated acute illness," the team write in their paper.
These vaccines have already saved millions of our lives. A massive study of more than 13 million US veterans suggests vaccines have significantly lowered both the risk of death and long COVID. But the price of their effectiveness is that people are becoming complacent.
Despite early signs a fourth COVID-19 vaccine dose provides a much-needed immunity boost, vaccination rates have already dropped drastically. So, unfortunately, the biggest challenges to ongoing widespread COVID-19 vaccinations continue to be psychological and social.
But everything we can do to encourage people to keep getting vaccinated will save lives.
"We hope that this finding would spur more studies to fully understand the underpinning mechanisms behind vaccine-associated side effects and collectively contribute to developing even more tolerable vaccines," Ooi concludes.
Their research was published in PLOS Biology.
