New research has made encouraging progress in tackling not one but two of the biggest problems facing our planet right now: plastic pollution and the use of fossil fuels as part of drug manufacturing processes.
Scientists from the University of Edinburgh in the UK have used Escherichia coli bacteria to convert molecules from the widely used polyethylene terephthalate (PET) plastic into the painkiller acetaminophen (also known as paracetamol).
Like a lot of drugs today, acetaminophen is mostly made out of fossil fuels. Switching those ingredients for waste products – like plastic – could offer an ingenious way of addressing two major environmental problems in one.
It's going to take a while to scale this up and prove it can be effective at an industrially and commercially viable level, so we shouldn't get too far ahead of ourselves, but there's a lot of potential in the new technology.
"This work demonstrates that PET plastic isn't just waste or a material destined to become more plastic – it can be transformed by microorganisms into valuable new products, including those with potential for treating disease," says biotechnologist Stephen Wallace from the University of Edinburgh.
The process starts by chemically degrading PET bottles. The resulting molecules are then fed to engineered E. coli, which use phosphate as a catalyst to convert the molecules into an organic compound containing nitrogen. Finally, these compounds are turned into the active ingredient of acetaminophen.
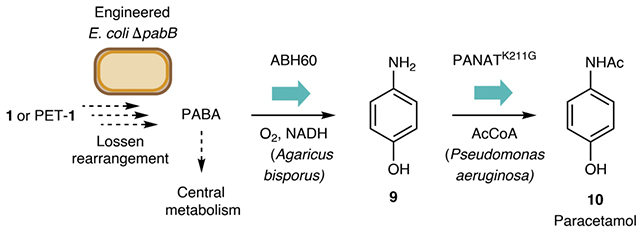
Among the numerous advantages of the process are that it can be completed in 24 hours in a compact laboratory setup, and that it works at room temperature, so there's no need for excessive heating or cooling. What's more, the team has managed to get it working at an impressively efficient 92-percent yield.
The reaction makes use of a well-established chemical reaction called the Lossen rearrangement, named after German chemist Wilhelm Lossen, who discovered it in 1872. Here, the reaction is made biocompatible so it can work in cells and living bacteria.
Related: Plastic-Eating Insect Discovered in Kenya Is The First of Its Kind in Africa
This was all done using PET bottles, but the plastic is also used extensively in food packaging, furniture, and manufacturing. This type of plastic is estimated to account for more than 350 million tons of waste per year, adding to the plastic pollution burden.
The same approach might also work for other types of bacteria and other types of plastic, according to the researchers, so there's potential here for more environmentally friendly recycling and drug production options.
It's a powerful example of how both natural and synthetic chemistry can be combined to find solutions to problems and drive innovation, and it may ultimately mean that E. coli plays a part in the production of our pain relief in the future.
"Nature has evolved an exquisite yet limited set of chemical reactions that underpin the function of all living organisms," write the researchers.
"By contrast, the field of synthetic organic chemistry can access reactivity not observed in nature, and integration of these abiotic reactions within living systems offers an elegant solution to the sustainable synthesis of many industrial chemicals from renewable feedstocks."
The research has been published in Nature Chemistry.