The first chemical reactions in the wake of the Big Bang have been recreated for the first time in conditions similar to those in the baby Universe.
A team of physicists led by Florian Grussie of the Max Planck Institute for Nuclear Physics (MPIK) in Germany has reproduced the reactions of the helium hydride ion (HeH+), a molecule made from a neutral helium atom fusing with an ionized atom of hydrogen.
These are the first steps that lead to the formation of molecular hydrogen (H2), the most abundant molecule in the Universe and the stuff from which stars are born. The new work, therefore, elucidates some of the earliest processes that gave rise to the Universe as we know it today.
Related: The First Molecular Bond in The Universe Has Finally Been Detected in Space

The birth throes of the Universe some 13.8 billion years ago produced a hot, dense soup of fundamental particles simmering at temperatures too high for atoms to form.
It took about 380,000 years for nuclei and electrons to lose enough energy to congeal into the very first elements. Those elements were the lightest the periodic table has to offer; about 75 percent hydrogen, 25 percent helium, and trace amounts of lithium.
Hydrogen continues to dominate the Universe's ingredient list today, as clouds of molecular gas that give birth to the stellar furnaces from which the heavier elements are born, either through fusion or violent explosions.
None of that, however, could happen without HeH+ – a molecule that scientists believe played a huge role in cooling the Universe enough so that the molecular clouds could contract enough to attain the density required to collapse under their own gravity to form the seeds of baby stars.
That's because HeH+ has a comparatively large separation between its positive and negative charges. In the presence of an electric field, a molecule with a large charge separation undergoes an energy shift that helps dissipate heat, which means HeH+ theoretically played a key role in paving the way for the formation of the first stars.
The researchers performed their experiments at the Max Planck Institute's Cryogenic Storage Ring, a facility designed to perform experiments in a vacuum environment at temperatures just a few degrees above absolute zero, around -267 degrees Celsius (-449 Fahrenheit), mimicking the conditions of deep space.
There, they carefully studied interactions between HeH+ and a hydrogen atom with one extra neutron in its nucleus, known as deuterium. An interaction between HeH+ and deuterium generates a neutral helium atom and a molecule consisting of one neutral hydrogen atom and one charged deuterium atom (HD+), with lower energy levels than the original components.
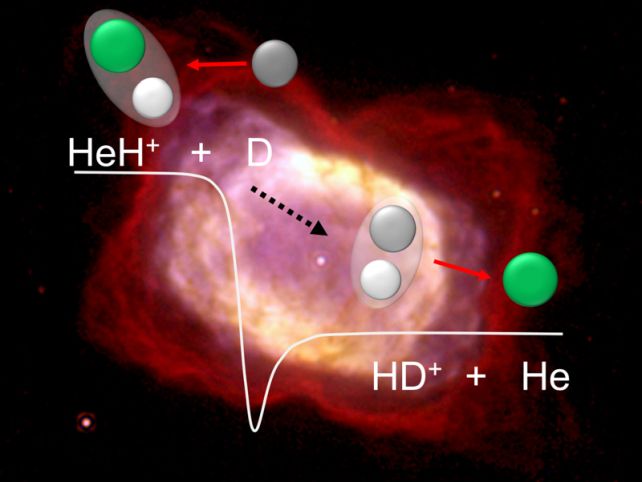
Within the storage ring, the researchers fired two beams of particles; one with HeH+ molecules, the other with neutral deuterium. They changed the speed of the two beams to alter the energy at which the particles collided as a proxy for temperature to see if temperature played a role in the reaction rate.
It did not. The rate at which the reaction took place remained steady, regardless of the proxy temperature – suggesting that the role HeH+ played in the early Universe did not decline as cooling unfolded, and that its role in the formation of the first generation of stars was a significant one.
"Previous theories predicted a significant decrease in the reaction probability at low temperatures, but we were unable to verify this in either the experiment or new theoretical calculations by our colleagues," physicist Holger Kreckel from the MPIK explains.
"The reactions of HeH+ with neutral hydrogen and deuterium therefore appear to have been far more important for chemistry in the early Universe than previously assumed."
The research has been published in Astronomy & Astrophysics.
