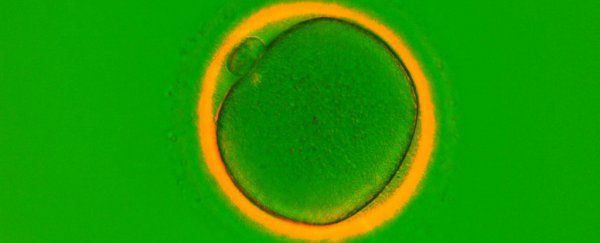
Women are well aware that the number of eggs they're born with is all they'll ever have, and when they inevitably run out, they can no longer have children. Hence the pressure of the biological clock.
But a new study has found the first evidence that women's ovaries might actually be able to grow new eggs. If confirmed, it could mean that post-menopause women and those with fertility problems might be able to conceive naturally after all.
To be clear, this is very early evidence taken from a small study involving cancer patients, and it needs to be replicated and explored further before we can say for sure what's going on.
But the possibility that adult women could grow new eggs would overturn our current understanding of reproductive biology, and the results have scientists very interested.
Here's what we know so far: researchers studying a small group of cancer patients found that women taking a chemotherapy drug called ABVD had a higher density of eggs than healthy women of the same age - suggesting that the drug could actually be encouraging their ovaries to grow new eggs.
"This was something remarkable and completely unexpected for us. The tissue appeared to have formed new eggs," lead researcher Evelyn Telfer from the University of Edinburgh in Scotland told Hannah Devlin in an exclusive for The Guardian.
"The dogma is that the human ovary has a fixed population of eggs and that no new eggs form throughout life."
But she added that we're still a long way from fully understanding what's going on.
"There's so much we don't know about the ovary," she said. "We have to be very cautious about jumping to clinical applications."
The team was inspired to conduct the study after noticing that the drug ABVD didn't seem to cause fertility problems in patients, unlike most other forms of chemotherapy.
To figure out what was going on, they looked at the ovarian biopsies of 11 women with Hodgkin lymphoma - eight of whom had been given ABVD and three who'd been given a stronger drug combination that's known to cause infertility.
They compared these samples to the ovarian biopsies of 10 healthy women.
Surprisingly, the women who'd been treated with ABVD had between double and four times the density of viable eggs in their ovaries than the healthy women. Those given the stronger drug combination had far fewer.
"It's a big increase, not just a few," Telfer told Devlin.
The eggs of the ABVD patients also appeared "younger", she notes, more like the eggs seen in pre-pubescent girls than adult eggs.
On the downside, the eggs of ABVD-treated patients didn't mature as well as the healthy women's - but the cancer patients who'd had ABVD didn't report any fertility problems.
This is a tiny sample size, so it's hard to make too many assumptions about what's going on without further study. But Tefler thinks the quantity and appearance of the ABVD patients' eggs suggests that they were newly grown from stem cells inside the ovarian tissue.
That said, David Albertini from the Centre for Human Reproduction in New York, who wasn't involved in the study, isn't so convinced.
"Honestly, I think there are too many other ways to explain the results, one of which is that new eggs were made," he told Devlin.
Some of those other explanations include the possibility that the extra eggs might have already been there, but rose to the surface during the treatment. Or maybe existing egg follicles split into two.
According to Nick Macklon, professor of obstetrics and gynaecology at the University of Southampton in the UK, who also wasn't involved in the study, the work "raises more questions than it answers".
"It's hugely controversial … This [new study] could be some evidence that it might be possible," he told Devlin, but adds that there's no evidence for clinical applications of this work.
Telfer and her team presented the research at the European Society of Human Reproduction and Embryology conference in Helsinki in July, and are currently undergoing review to publish the results in a journal.
Until further research is done, we really won't know what's going on. But it's definitely tantalising evidence that could change the way we think about women's reproduction forever.
"It suggests that the ovary is indeed a more complex and versatile organ than we have been taught, or that we expected, with an inherent capacity of renewal," Kenny Rodriguez-Wallberg from the Karolinska University Hospital in Stockholm, who wasn't involved in the study, told The Guardian.