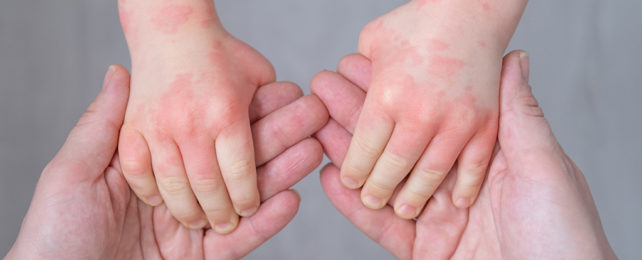
Eczema (or atopic dermatitis) affects millions of people, particularly children under the age of six.
The chronic inflammatory skin disorder causes the skin to go red and dry and to start oozing and itching, making life very uncomfortable.
There's currently no cure for the condition, just ways of managing it – but an existing drug is incredibly effective at reducing the signs and symptoms of eczema in kids under six with moderate to severe cases of the disorder.
It's the first time a complex biologic drug like this has been tested on this age group.
The drug in question is dupilumab. In a new study, 162 North American and European kids between the ages of 6 months and 6 years with moderate-to-severe eczema were given dupilumab or a placebo across the course of 16 weeks.
More than half of the kids given the drug showed a 75 percent reduction in symptom severity. Itchiness was significantly reduced, and the kids could sleep much better.
"Preschoolers who are constantly scratching, awake multiple times a night with their parents, irritable and markedly curtailed in their ability to do what other children their ages can do, improved to the extent that they sleep through the night, change their personalities and have a normal life – as babies and children should," says dermatologist Amy Paller from Northwestern University in Illinois.
Dupilumab targets an important immune inflammation pathway in allergies and is already used to treat eczema in older children and adults, as well as asthma, nasal polyps, and other allergy-mediated problems.
Until now, it hadn't been approved as safe or confirmed to be effective for those under the age of six – around 19 percent of this demographic are thought to have eczema, while 85-90 percent of those who develop eczema in their lives see the first signs of it before the age of five.
Around a third of this age group with eczema have a moderate to severe case of the disorder, with accompanying itchiness that is debilitating: These kids can't sleep properly, which has all kinds of knock-on effects and consequences.
While immune-suppressing medications such as oral steroids are often used for severe cases of eczema, there are concerns over their suitability for young children – both in terms of the short-term side effects and the long-term health complications, according to Paller.
"The group in whom we worry the most about safety – those under five – had not been tested and were unable to get [dupilumab]," says Paller. "The effect for most of these younger children is dramatic and at least as good as we've seen with the risky immunosuppressant medications."
Dupilumab already has a safety profile marked as "outstanding", and no further laboratory tests are necessary. It's now available to children as young as 6 months, and either a parent or healthcare professional can administer the drug through a monthly shot.
Moreover, the researchers think it could also have preventative effects. Because it takes such an aggressive approach to calming the immune system's inflammation response, there's a good chance it might also protect against other allergic issues developing later in life.
Dupilumab might even prove useful in dealing with other health issues in younger kids, the researchers suggest – although further studies are going to be necessary to establish how else it could be effective.
"The ability to take this drug will significantly improve the quality of life for infants and young children who suffer tremendously with this disease," says Paller.
"Atopic dermatitis or eczema is so much more than just itchy skin. It is a devastating disease. The quality of life of severe eczema – not only for the child but also parents – is equivalent to many life-threatening diseases."
The research was sponsored by Regeneron Pharmaceuticals and Sanofi, who jointly developed dupilumab, and the study has been published in The Lancet.