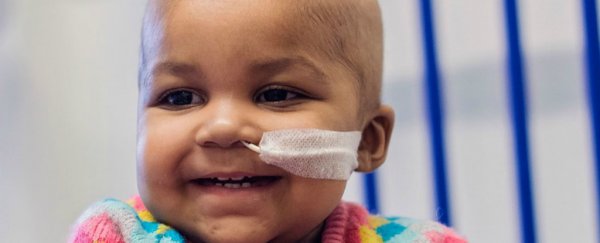
Researchers in the UK have successfully treated Layla, a baby girl with leukaemia, using a gene-editing therapy that had previously only been trialled in mice. Doctors say it's too soon to declare her cured, but she's now living healthily and cancer free.
The therapy involved using mass-produced gene-edited cells to attack the leukaemia in her body, and it was the first time it had been tried in a human. But because Layla's case had been declared incurable and doctors had exhausted all other options, her parents were granted permission to use the experimental treatment on compassionate grounds.
Layla's results will be presented at the American Society of Hematology in December, and represent the first time that a human life has been saved by gene-editing – despite many years of promising trials using the technique.
"As this was the first time that the treatment had been used, we didn't know if or when it would work and so we were over the moon when it did," said Paul Veys, Layla's lead clinician at the Great Ormond Street Hospital (GOSH) in London. "Her leukaemia was so aggressive that such a response is almost a miracle."
While this is only one case study, what's really exciting is that it's the first evidence that using edited versions of generic, non-personalised cells could work as an effective therapy, paving the way for more affordable and accessible treatment options for a range of cancers.
Layla was born in the UK, and was diagnosed with what's known as acute lymphoblastic leukaemia – an aggressive cancer of the bone marrow – at just three months old. She underwent several rounds of intense chemotherapy, a bone marrow transplant, and took part in an experimental trial all before her first birthday, but the cancer came back.
That's when her doctors heard about a new type of gene editing being tested at University College London. Normally gene editing works by taking immune T-cells from a patient's body and then genetically engineering them to attack cancerous cells before placing them back in the body.
This is already being trialled in humans, and results so far suggest that it can be effective. But it's also incredibly expensive and time consuming, and it won't work for patients like Layla, who have already undergone extensive treatment for leukaemia and don't have enough healthy T-cells left to work with.
Instead the researchers at University College London, led by Waseem Qasim, have been working on an 'off-the-shelf' therapy, which could be quickly and cheaply given to patients without having to first collect their cells.
In this approach, a healthy person donates a whole bunch of T-cells, which are then modified to make sure they're safe to transfer, creating what the researchers call UCART19 cells.
To create these cells, Qasim and his team use a pair of 'molecular' scissors, known as TALEN proteins, to switch off certain receptors, ensuring that the UCART19 T-cells only attack leukaemia cells, and not healthy ones. They also removed genes to make the cells invisible, so that they wouldn't be destroyed by other leukaemia drugs.
"The approach was looking incredibly successful in laboratory studies," said Qasim, "and so when I heard there were no options left for treating this child's disease, I thought 'why don't we use the new UCART19 cells?'"
Layla was given 1 ml of the cells, and then spent the next weeks in isolation while the T-cells attacked her cancer cells. Two months later, once the doctors confirmed that the leukaemia cells had all been removed, she was given another bone marrow transplant.
She's now recovering well at home and is having regular check ups. And the best part is that once the final bone marrow transplant was done, Layla's own immune system kicked back in and destroyed the UCART19 cells, so there was no trace left of the gene-editing.
The researchers have now scheduled a proper clinical trial of the UCART19 cells, funded by biotech company Cellectis, to start in early 2016. In addition to making sure the treatment works, these trials will ensure that the modifications made to the T-cells can't be passed on, and aren't toxic to a wider group of patients.
"We have only used this treatment on one very strong little girl, and we have to be cautious about claiming that this will be a suitable treatment option for all children," said Qasim. "But, this is a landmark in the use of new gene engineering technology and the effects for this child have been staggering. If replicated, it could represent a huge step forward in treating leukaemia and other cancers."