Giant whales have little cancer relative to their body size. It's a biological mismatch known as Peto's paradox, which describes how large, long-lived animals, despite having trillions more cells than humans or tiny critters, don't develop more cancers.
Cancer is a disease of runaway cell division, where genetic mutations drive cells to divide and divide, forming masses called tumors. So you would think that the larger an animal is, the more cells it has, and the greater chance those cells have to accumulate genetic errors that lead to cancer, especially over a long lifetime.
But renowned British statistician Richard Peto noticed when comparing mice and men in the late 1970s that this wasn't the case. Subsequent studies showed that across creatures great and small, cancer doesn't become more common the more cells a species has. Elephants, like whales, don't have a ton of cancers.
It might sound like biologists are hung up on a decades-old paradox that's just a quirk of nature – but it's an important quirk. Resolving this paradox may help develop new strategies to prevent or suppress cancer in people.
Now a team of researchers from the University of Rochester in New York has found a solution to the paradox in bowhead whales, Balaena mysticetus, the second-largest but longest-lived animal on Earth.
"By studying a mammal capable of maintaining its health and avoiding death from cancer for over two centuries," write biologist Denis Firsanov and colleagues in their preprint paper, "we are offered a unique glimpse behind the curtain of a global evolutionary experiment that tested more mechanisms affecting cancer and aging than humans could ever hope to approach."
In a series of lab experiments, the researchers found that bowhead whale cells are better at repairing DNA damage than cells from humans, mice, and cows. Whales, it seems, nip DNA damage in the bud "with uniquely high efficiency and accuracy compared to other mammals," Firsanov and colleagues write.
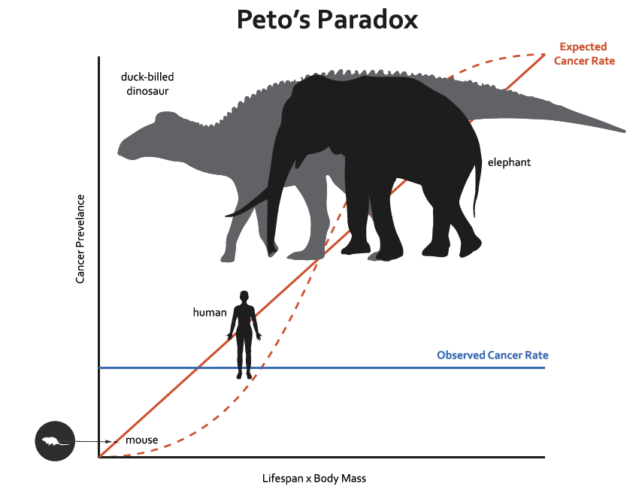
Put simply, bowhead whales can tolerate more hits to their genomes because they have a sharply tuned, quick-fix system for repairing DNA damage. The researchers found that in one region of DNA that whales, humans, mice, and cows all share, whale cells were more likely to repair DNA breaks (snipped by CRISPR) without errors.
Bowhead whale cells also pumped out a DNA repair protein called CIRBP at much higher levels than the other species studied. And when lab-grown human cells were engineered to produce bulk CIRBP, this genetic tweak boosted their ability to repair DNA error-free.
"This strategy that does not eliminate cells but repairs them may be critical for the long and cancer-free lifespan of the bowhead whale," Firsanov and colleagues conclude.
Yale University cancer biologist Jason Sheltzer, who was not involved in the work, says the "fascinating" preprint – which has not yet been peer-reviewed – "provides a new model for how big animals avoid cancer".
"Maybe they're just better at DNA repair than us?" Sheltzer mused on Twitter. "As a next step," he added, "I'd love to see this validated in an animal model – if you drive high expression of whale CIRBP [protein] in the mouse, are they cancer-resistant?"
Of course, as past research shows, translating a discovery like this into cancer therapy isn't easy.
When scientists discovered in 2015 that elephants have extra copies of a tumor-suppressor gene called TP53, the next logical step was to test whether increasing the activity of TP53 in mice also tamps down cancer. Tumor suppressor genes effectively 'blow up' any cells they find harboring too much DNA damage, and it turns out elephants have very low thresholds for waging war on damaged cells.
However, overexpressing a form of the TP53 protein in mice, while it suppressed cancer, also induced premature aging in the animals. Other studies may have found a workaround – and scientists keep looking for other possibilities.
"There are likely many solutions to Peto's Paradox in nature because large body size has evolved independently so many times across the history of life," remarked Marc Tollis, a geneticist at Northern Arizona University, in a 2017 paper with two other researchers.
In other words, each long-lived or large animal, from naked mole rats to African elephants, has evolved its own ways of suppressing cancer that scientists are itching to figure out.
Other explanations could be that tumors in large animals are slow growing and less lethal or that big animals have better immune surveillance. However, these solutions have not yet been observed in large-bodied species and need more research.
"Every time we discover a potential mechanism for cancer suppression in a species, there is the chance that we can find new therapeutic targets and approaches to cancer prevention to save human lives," Tollis and colleagues write. But no doubt it will require "substantial effort to translate recent discoveries into effective therapies for humans."
The University of Rochester study is available on the preprint server biorXiv before peer review.
