A rare and mysterious slime mold may contain clues about the emergence of multicellularity.
Scientists have found that the typically single-celled species Fonticula alba joins together to form an invasive whole that tears through a bacterial colony at a specific stage of its life cycle, feasting like a single organism. This collective has animal-like properties and behaves like an aggressive cancer.
This discovery could help us to understand how multicellular organisms emerged from the simple, single-celled origins of all life on Earth.
Fonticula alba is a rare and peculiar type of slime mold. It was isolated in 1960 from a sample found in dog feces in Kansas; its life cycle and behavior were subsequently studied in a laboratory where it was cultured.
Like many other slime molds (which are not actually fungi, but members of the protist kingdom, a sort of catch-all group for anything that can't be neatly categorized as animal, vegetable, or fungus), F. alba spends most of its life cycle as a single-celled organism, feeding on bacteria as part of the cycle of decay.
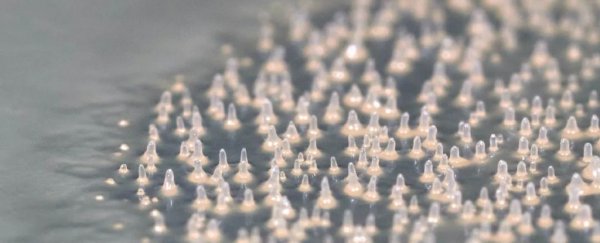
When it comes time to propagate, these single cells aggregate, coming together in a multicellular form to grow volcano-like fruiting bodies that release spores to grow… more F. alba.
It's also unlike other slime molds. F. alba is more closely related to fungi than other slime molds and is categorized under the same clade as fungi.
A team of researchers led by biologist Christopher Toret of the University of Geneva in Switzerland wanted to learn more about the organism's little-studied life cycle, so they set about culturing it in the lab. Although the slime mold can feed on different types of bacteria, a common fecal bacterium called Klebsiella pneumoniae was identified as the optimal co-culture for F. alba back in 1979, so that is what the researchers used.
They grew colonies of K. pneumoniae, and introduced the slime mold at different stages of the bacteria's life cycle. The researchers found something very peculiar and unexpected: towards the end of the bacteria's life, as K. pneumoniae were running out of food, F. alba aggregated into a multicellular state – not to fruit, but to feed on the bacteria.
As the slime mold came together and moved into the bacterial colony, it developed transient cell columns, forming tentacle-like filaments; these were similar to the hyphae we know from fungi which perform a number of functions, including seeking nutrient sources. These filaments performed cooperative search and invasion of the bacterial agar plate to seek out new sources of food.
The slime mold cells came together to form these filaments, with a single 'leader' cell at the tip, and 'follower' cells arranged behind. Somehow, these cells communicate, with the leader cell issuing information to the follower cells.
When the researchers disrupted the leader cell in a tendril using a laser, the follower cells fell into disarray, no longer able to seek out new food sources. The same disarray was not observed when one of the follower cells was disrupted. This suggested that the cells indeed had specific roles in this multicellular state.
This suggests, the researchers said, a previously unconsidered origin for fungal hyphae.
"We suggest a hypothesis where hyphae could have had a direct aggregative origin," the researchers wrote in their paper.
"The last common ancestor of fungi and F. alba may have assembled amoeboid cells into head-to-tail arborized invasive collectives."
Cancer states also use leader-and-follower dynamics for invasion, suggesting that different kinds of cells can display similar behaviors for different reasons. This means F. alba could be a powerful model organism for understanding the emergence of multicellularity as a general concept, the team said.
The research has been published in Current Biology.
H/T: The Scientist