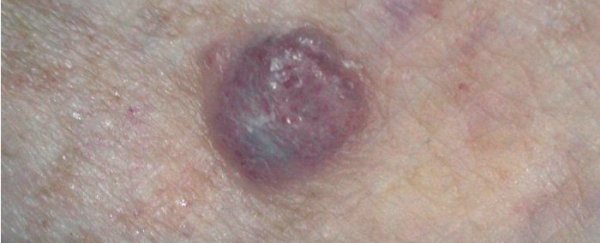
An Australian-led clinical trial has shown that a new type of immunotherapy drug called Keytruda helps patients with advanced melanoma skin cancer live longer with fewer side effects, compared to the standard medication.
Publishing in The New England Journal of Medicine, the researchers report that more than 70 percent of patients who were given Keytruda lived for one year, compared to only 58 percent of those given the current treatment.
"The results are world-first, where two effective immunotherapies are directly compared, and we significantly improve outcomes for patients even further," the leader of the study Georgina Long, from the Melanoma Institute of Australia, told ABC's Sophie Scott. One Australian dies from melanoma every six hours, so it's a much-needed development. "This really is important as this new drug works much better. To show such a large improvement is outstanding."
Keytruda works by binding to a cell surface protein known as PD-1, which allows the body's own immune system to detect the tumour cells and wipe them out. The drug was trialled head-to-head against another immunotherapy called Yervoy, which is the current standard melanoma treatment.
The trial involved 834 patients from 16 countries, whose melanoma had spread to more than one part of the body. One group of patients was given Keytruda every two weeks, another was given the drug every three weeks, and a final group received the standard four cycles of Yervoy.
The participants were revisited one year later, and 74 percent who received Keytruda every two weeks were still alive, as were 68 percent of those who had received the drug every three weeks. By comparison, only 58 percent of those who received Yervoy were still alive.
The rates of side effects, which include the bowel disease colitis, were also lower in the patients who had taken Keytruda.
Based on the promising results, the Australian Therapeutic Goods Administration has now registered Keytruda as suitable for the treatment of patients with advanced melanoma. The scientists are now hoping it will be listed on the country's Pharmacuetical Beneifts Scheme, which will help subsidise the price of the treatment, which can cost around A$150,000 a year.
In the US, the drug is already approved by the Food and Drug Administration as a second-line of defence if Yervoy doesn't work. But, off the back of the new results, researchers are hoping it will be approved as a first-line treatment. The drug creators, Merck & Co, are also trialling Keytruda on lung cancer, with promising results.
However, the team is now curious to find out why roughly 30 percent of the patients didn't respond to Keytruda. "This is not a panacea and for everyone," Long told Scott. "We are now looking at why those patients don't respond."
Just last week, researchers in the US announced another new immunotherapy drug that is capable of stopping the growth of melanomas in up to 90 percent of the mice tested. While that's a long way off human trials, it's promising to know that in the future we may have a range of options to help us fight this devastating disease.
Sources: ABC, The Wall Street Journal