Severe hair loss from the autoimmune condition, alopecia areata, could be reversible in some patients if they take a daily oral drug for several months.
A recent randomized, double-blind clinical trial on the medication has shown such promising results, the United States Food and Drug Administration (FDA) has just approved its use for patients 12 years and older.
Alopecia is marked by hair loss on the scalp, face, or body. It occurs when the immune system begins attacking a person's own hair follicles, and while most patients are otherwise healthy, patchy or complete losses of hair can have serious mental and emotional impacts.
Some patients with severe forms of the disease lose all of their scalp hair, eyelashes, eyebrows, and all the rest of their body hair as well, a condition known as alopecia universalis. Such severe cases tend to be especially resistant to available treatments, but a new drug called ritlecitinib could help change that.
In stage two and three clinical trials, the oral medicine reversed up to 80 percent of hair loss on the scalp for close to a quarter of all patients, and so far appears to be one of the only treatments for severe alopecia that is both effective and well-tolerated by a significant number of people. Its approval also makes it the only treatment available for children.
Another oral drug for alopecia, called baricitinib, was approved by the FDA in 2022 to exclusively treat adults. It works about 20 percent of the time at the recommended dosage.
In the coming weeks, ritlecitinib will be available to patients as a much needed alternative. It's being sold by Pfizer under the brand name LITFULO™, and the recommended dosage is 50 milligrams a day.
"While patients may start to develop symptoms of alopecia areata at any age, most people start showing signs in their teens, twenties, or thirties," says dermatologist Brittany Craiglow from Yale University in a press release from Pfizer.
"LITFULO is a particularly important treatment option for younger patients with substantial hair loss, who often struggle with such a visible disease."
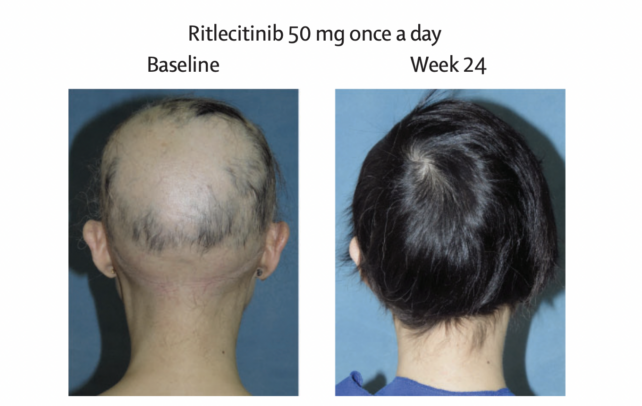
In the recent clinical trial on LITFULO, more than 700 patients with alopecia were enrolled from 18 nations. Every participant had experienced hair loss on half their scalp for less than a decade, and half the cohort had experienced total scalp hair loss.
Compared to those who took a placebo, patients who took 50 milligrams of LITFULO a day showed significant hair regrowth. In total, about 23 percent of patients treated with the drug for six months showed 80 percent or more scalp hair coverage compared to 1.6 percent of those who took a placebo.
Those patients who had had the disease for longer tended to show poorer outcomes, which suggests the drug is most effective in the early stages of an alopecia flare-up, which can last months or up to a year at a time.
The exact way in which LITFULO reverses hair loss and promotes hair growth is still somewhat unknown, but in mouse models and scalp biopsy studies, it seems to dampen the body's overactive immune response.
As an enzyme inhibitor, the drug stops immune cells from initiating a specific signaling pathway that can cause inflammation in the hair follicle.
The regrowth of hair observed in clinical trials suggests these hair follicles can be restored, possibly by reinstating their 'immune privileges'.
Unfortunately, this mechanism of action can impact the immune system's usual function, meaning patients on LITFULO can be more susceptible to infections and sicknesses.
In the current clinical trial, side effects were deemed minimal and manageable.
"LITFULO is an important treatment advancement for alopecia areata, an autoimmune disease that previously had no FDA-approved options for adolescents and limited options available for adults," says Pfizer's chief commercial officer Angela Hwang.
"With today's approval, adolescents and adults who struggle with substantial hair loss have an opportunity to achieve significant scalp hair regrowth."
The study was published in The Lancet.
