Alzheimer's disease affects the brain in tragic and seemingly irreversible ways, but a new study gives hope that its neurological damage may not be entirely beyond repair.
US researchers found a drug candidate called P7C3-A20 returned cognitive functions to mice with models of Alzheimer's disease. Brain cell damage was halted, inflammation was reduced, and the blood-brain barrier (the brain's protective shield) was also restored.
"We were very excited and encouraged by our results," says neuroscientist and psychiatrist Andrew Pieper, from Case Western Reserve University.
Related: Mouse Study Suggests Nose-Picking Has a Surprising Link With Alzheimer's
P7C3-A20 was selected as a neuroprotective compound known to restore the balance of NAD+ (nicotinamide adenine dinucleotide). This molecule helps cells break down compounds for fuel and build functional proteins.
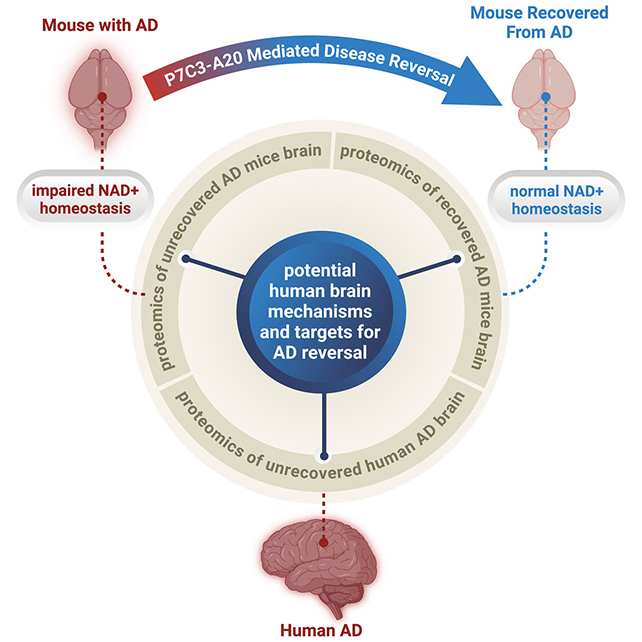
Previous studies in animals had found that restoring levels of NAD+ with compounds such as P7C3-A20 can improve signs of Alzheimer's disease. In fact, some of the same researchers have already demonstrated that P7C3-A20 and its NAD+ boosting powers can repair mouse brains after head injuries.
In this follow-up study, NAD+ levels returned to normal in mice with advanced symptoms after six months of daily injections. Related biomarkers for inflammation and DNA damage also indicated there was sufficient NAD+ for cells to function normally again.
The team tested two mouse models of Alzheimer's, each related to one of the disease's two hallmark pathologies – amyloid-beta protein plaques and tau protein tangles.
It's been theorized that these abnormal clumps of protein have a role in the death of neurons and brain function disruption in Alzheimer's, though the relationship has been unclear. While P7C3-A20 restored brain function, it didn't clear out these plaques and tangles, suggesting that cells may be able to deal with the aggregated proteins if they are firing on all cylinders.
"Restoring the brain's energy balance achieved pathological and functional recovery in both lines of mice with advanced Alzheimer's," says Pieper.
"Seeing this effect in two very different animal models, each driven by different genetic causes, strengthens the new idea that recovery from advanced disease might be possible in people with AD when the brain's NAD+ balance is restored."
There is a long way to go before we know whether treatments like this one can repair and reverse the damage of Alzheimer's disease in humans. That will require more animal studies and carefully designed clinical trials. However, this is a clear sign that NAD+ could be a crucial factor in future treatments.
Related: Microdosing Cannabis Pauses Alzheimer's Decline in Unprecedented Trial
Those treatments will have to be carefully controlled and calibrated, as an overload of NAD+ has been linked to cancer in the past. And with a disease as complex as Alzheimer's, it's likely that a complex solution is needed.
"The key takeaway is a message of hope – the effects of Alzheimer's disease may not be inevitably permanent," says Pieper. "The damaged brain can, under some conditions, repair itself and regain function."
The research has been published in Cell Reports Medicine.