Pregnancy loss remains common around the world. About 15 percent of known pregnancies end in miscarriage, although the true number is likely much higher, since many pregnancies are lost before they are discovered.
In a new study, researchers uncovered key information about genetic factors related to aneuploidy, or an abnormality in the number of chromosomes in a cell – one of the most common causes of pregnancy loss.
A miscarriage can occur for a variety of reasons, but chromosomal abnormalities are a common factor. About half of all known miscarriages in the first or second trimester result from fetuses possessing too many or too few chromosomes.
To investigate the underpinnings of aneuploidy, researchers analyzed genetic data from nearly 140,000 in vitro fertilization (IVF) embryos, offering new details about how common genetic variations can increase some parents' risk for pregnancy loss.
"This work provides the clearest evidence to date of the molecular pathways through which variable risk of chromosomal errors arises in humans," says senior author Rajiv McCoy, a computational biologist at Johns Hopkins University.
"These insights deepen our understanding of the earliest stages of human development and open the door for future advances in reproductive genetics and fertility care," McCoy says.
Chromosomal abnormalities typically arise in the egg, with a frequency that correlates positively with a mother's age. While age is a known risk factor, our understanding of the broader genetic context has been hindered by limited data, the researchers note.
To remedy that, scientists would need to analyze a huge volume of genetic data from many thousands of embryos before pregnancy loss, along with data from their biological parents.
"This is a trait closely related to survival and reproductive success, so evolution will only allow genetic differences with small effects to be common in the population," McCoy says. "You need large samples to be able to detect these small effects."
The researchers used clinical data from pre-implantation genetic testing of IVF embryos, analyzing 139,416 embryos from 22,850 sets of biological parents in hopes of finding patterns. They identified 92,485 aneuploid chromosomes within 41,480 different embryos.
"Here the power comes from these huge sample sizes," McCoy says. "That allowed us the scale and resolution to discover several of the first well-characterized associations between the mom's DNA and her risk of producing embryos that will not survive."
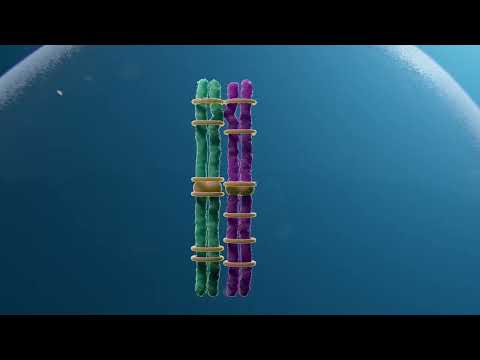
The strongest of these associations was found in genes that help influence the way chromosomes pair, recombine, and gather together during the process of meiosis in egg cell lines.
A variant of the gene SMC1B, which encodes a protein that helps hold chromosomes together during meiosis, was associated with reduced crossover counts and with increased maternal meiotic aneuploidy, the study found.
The analysis also highlighted associations with several other genes involved with crossover recombination, including C14orf39, CCNB1IP1, and RNF212.
"This finding is especially compelling," McCoy says, "because the genes that emerged from our study in humans are exactly the ones that experimental biologists have detailed over decades as critical for recombination and chromosome cohesion in model organisms like mice and worms."
Female meiosis initially occurs during fetal development, as chromosomes pair and recombine, then stops for years until it resumes later in life for ovulation and fertilization.
Genetic variations may lead to problems in the interim, resulting in chromosomes that separate too readily, and thus potentially setting the stage for aneuploidy when meiosis resumes.
"Our results demonstrate that inherited differences in these meiotic processes contribute to natural variation in risk of aneuploidy and pregnancy loss between individuals," McCoy says.
Predicting individual risk for pregnancy loss will remain difficult despite these findings, the authors point out, given the importance of additional factors beyond genetics, such as maternal age and environmental exposures.
Nonetheless, understanding these genetic factors could be valuable for drug development and provides a foundation for future research into both maternal and paternal genetic variations related to pregnancy loss.
The study was published in Nature.

