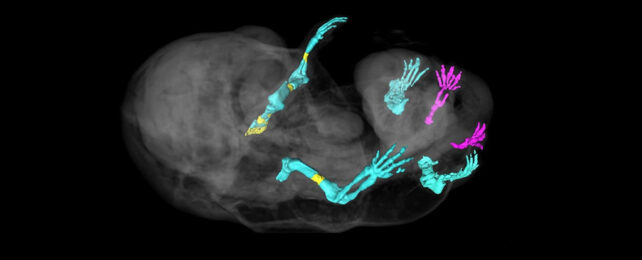
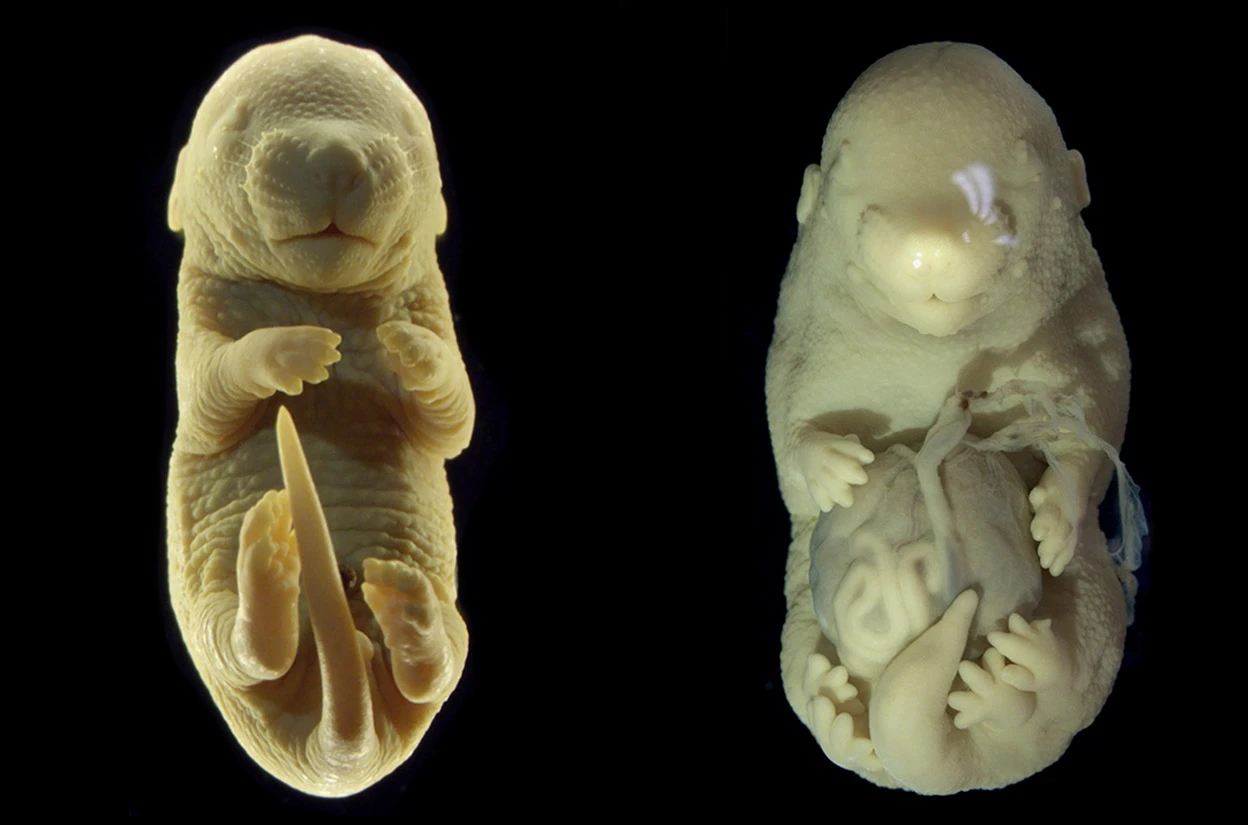
Turning off a gene early in mouse development led researchers to end up with an accidental six-legged embryonic mammal.
This strange result took the spinal cord research of developmental biologists Anastasiia Lozovska and Moisés Mallo and their colleagues at Portugal's Gulbenkian Science Institute in a new direction.
"I didn't choose the project, the project chose me," Mallo told Sara Reardon at Nature News.
The team compared 10 to 17-day-old mouse embryos with and without functioning versions of the gene in question, Tgfbr1, which codes for the Tgfbr1 receptor protein.
Tgfbr1 contributes to a signaling pathway that gives a forming body its trunk-to-tail directions. This pathway provides a 'create a hindlimb' here, or 'external genitals' instructions to the developing embryo's cells.
As a mammalian embryo grows, it builds structures sequentially from head to tail. Early in development, genetic mechanisms shift from focusing on the head to extending the body and laying the foundations for the major organ systems.
Later, a second transition occurs where the activation of genes in multiple layers of tissue extends the trunk to create a tail.
It's during this process that interactions between newly emerging tissues generate structures necessary for the body's exit channels and genitalia.
While legs and arms share many of the same genes, this early in the process, the hindlimbs and genitals have more in common. There has been some suggestion they arise from the same initial primordium structure in ancestral species.
"It will be therefore interesting to determine whether a mechanism related to the developmental plasticity uncovered by our work… could help [explain] the absence of hindlimbs in snakes but their presence in most lizards," Lozovska and colleagues wonder.
The scientists found that despite the rather dramatically different placement of the extra legs in the embryos without a functioning version of Tgfbr1, the other genes expressed in these legs are similar to those found in normal mouse limbs.
Both appendages arise from the middle of three tissue layers that compose an early embryo, the mesoderm. As the cells turning into the limbs push out into the surrounding outer layer of tissues (endoderm) it is possible they then receive more 'become legs' messaging to further their development into malformed but mature limb structures, the researchers suspect.
Lozovska and team took a closer look at the DNA in the mutant leg tissue compared to the control mice and identified chromatin remodeling – the proteins that control access to the cells' DNA had been switched to 'legs' configuration, instead of the 'genital' configuration.
The researchers still don't know the exact mechanism between the Tgfbr1 gene suppression leading to an extra pair of legs.
Understanding more about these fundamental processes will equip researchers with additional tools to tackle developmental challenges and diseases.
This research was published in Nature Communications.