The surprising force that holds our DNA together may also be responsible for changing its shape to allow for its repair, copying and gene shuffling, according to new experimental evidence.
First discovered back in the 1950s, the double helix structure of our genetic material has since become iconic. Appearing like a twisted ladder, the rungs in the middle are nitrogen base-pairs, held together by hydrogen bonds.
Connecting both sides, the role of these links in keeping our DNA together has been viewed as a "fundamental paradigm", but there's another important factor at play, and it's to do with hydrophobic, or water-repelling bonds.
DNA replication occurs through the help of several enzymes, which 'unzip' DNA molecules by breaking their hydrogen bonds. It turns out, however, that's not the only way to destabilise the double helix.
By testing the DNA in an environment more hydrophobic than normal, researchers at Chalmers University of Technology in Sweden have now shown for the first time that this water-repelling force can be used to unravel the double helix.
By gradually adding a semihydrophobic solution of polyethylene glycol - which is often used in cars as antifreeze - the team has shown that DNA loses its structure, and that this happens right as its surroundings go from water-loving to water-repelling.
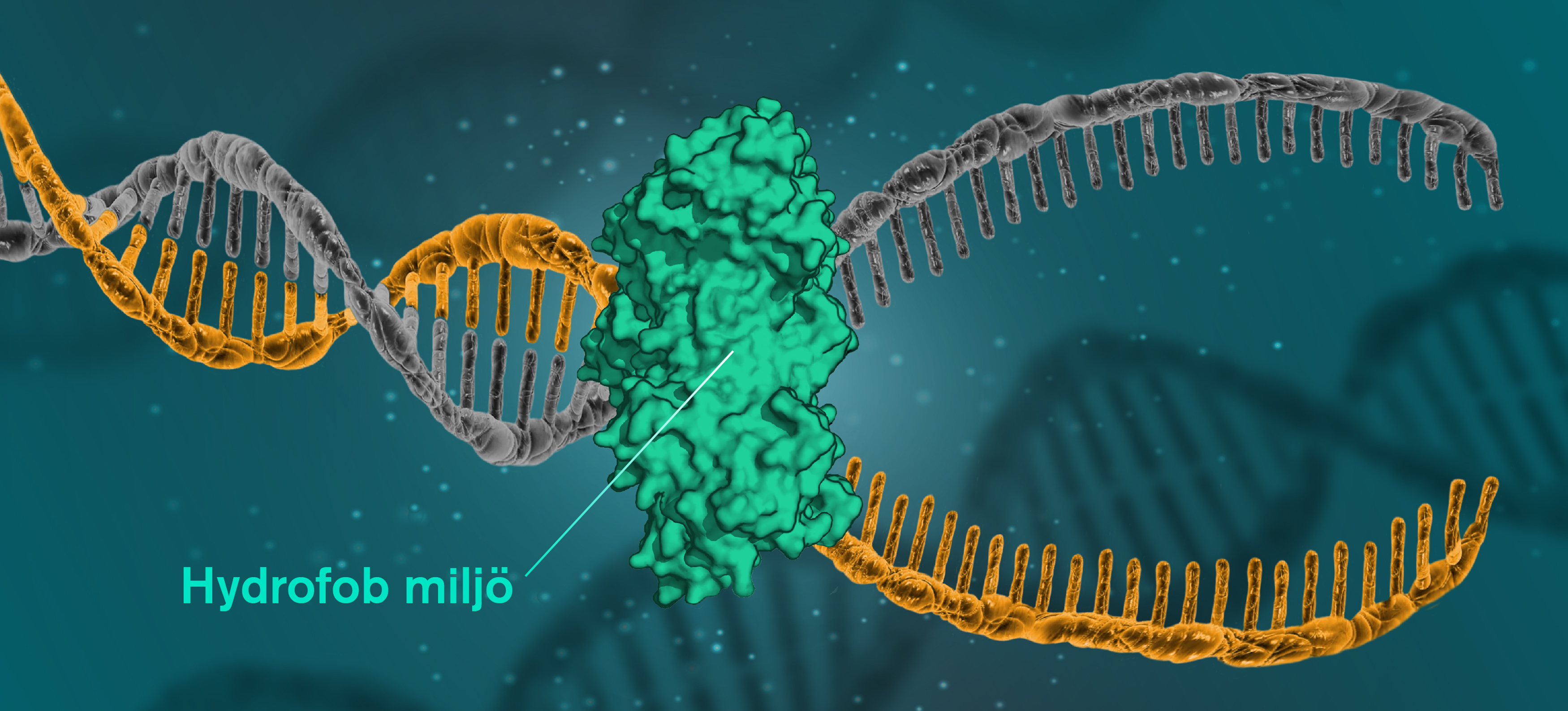 (Yen Strandqvist/Chalmers University of Technology)
(Yen Strandqvist/Chalmers University of Technology)
It's unclear if enzymes in nature do something similar, but given previous research on the topic, the team thinks this is a possibility.
"Cells want to protect their DNA, and not expose it to hydrophobic environments, which can sometimes contain harmful molecules," explains chemical engineer and lead author Bobo Feng.
"But at the same time, the cells' DNA needs to open up in order to be used. We believe that the cell keeps its DNA in a water solution most of the time, but as soon as a cell wants to do something with its DNA, like read, copy or repair it, it exposes the DNA to a hydrophobic environment."
Steven Brenner, a molecular biophysicist at NASA, told ScienceAlert this was an important discovery that demonstrates a new way enzymes might 'melt' the double helices of DNA for transcription or repair. Still, he warns, the way in which many media outlets have been covering this paper is not entirely accurate.
Despite what many are reporting, he says the results do not suggest hydrogen bonds are unimportant for DNA formation, while hydrophobic forces are.
In fact, this is hardly a new idea. According to Brenner, models that include hydrophobic interactions in the double helix date back to at least the 1990s, and today, there are whole research programs built around this concept.
In 1997, scientists further tested the notion that hydrogen bonds alone can keep the two strands of a DNA double helix together. That textbook explanation, it seemed, was inadequate, and several years later, in 2004, a study found hydrogen bonding was not required for the stability of base pairs.
Just a few years ago, in 2017, a study showed that a lack of complementary hydrogen bonds doesn't really bother cells, and that synthetic bases are successfully transcribed and translated anyway, using only hydrophobic forces.
"It would be very easy to say complementary hydrogen bonds are what define DNA and RNA," said biochemist Floyd Romesberg, one of the authors of the 2017 study.
"But we've found that forces other than hydrogen bonding can productively participate in every step of information storage and retrieval."
Yet as much as we've learned over the years, there are still limits to the conclusions we can draw from these models.
"One of the sad lessons of physical organic chemistry from the last century," Benner told ScienceAlert, "is that efforts to separately model the behaviour of molecules as the consequence of different factors .. tells you more about the chemist doing the modelling than it tells you about the molecules themselves."
These frameworks, for instance, can either be evaluated on their ability to merely explain DNA or on their ability to actually make it. Personally, Benner believes the latter analysis is more objective, because explanations on their own can often just convince us we understand what's going on.
"If, however, our models actually allow us to make things, then they must really have some reality behind them," he argues.
In the end, Benner says both hydrogen bonding and hydrophobicity have proved necessary to make natural DNA, and this double-pronged model is currently used both in human medicine and in NASA's search for alien life.
The new research adds to this idea by providing a possible biological mechanism for this process.
"Nobody has previously placed DNA in a hydrophobic environment like this and studied how it behaves, so it's not surprising that nobody has discovered this until now," says Feng.
The findings were published in PNAS.
Editor's note (26 Sep 2019): A previous version of this article made erroneous claims about the role of hydrogen bonds in the structure of DNA. These concepts have now been clarified to more accurately reflect the research.