A newly observed isotope of oxygen is defying all our expectations for how it should behave.
It's oxygen-28, with the highest number of neutrons ever seen in the nucleus of an oxygen atom. Yet, while scientists believe it should be stable, it decays rapidly – calling into question what we thought we knew about "magic" numbers of particles in the nucleus of an atom.
The nucleus of an atom contains subatomic particles called nucleons, consisting of protons and neutrons.
The atomic number of an element is defined by the number of protons it has, but the number of neutrons can vary.
Elements of different neutron numbers are known as isotopes; oxygen has 8 protons, but can have differing numbers of neutrons.
Previously, the largest number of neutrons observed was 18, in the oxygen isotope oxygen-26 (8 protons plus 18 neutrons equals 26 nucleons).
Now, a team led by nuclear physicist Yosuke Kondo of Tokyo Institute of Technology in Japan has found two oxygen isotopes that we've never seen before, oxygen-27 and oxygen-28, with 19 and 20 neutrons respectively.
The work was performed at the RIKEN Radioactive Isotope Beam Factory, a cyclotron accelerator facility designed to produce unstable isotopes.
First, the team fired a beam of calcium-48 isotopes at a beryllium target to produce lighter atoms, including fluorine-29, an isotope of fluorine with 9 protons and 20 neutrons.
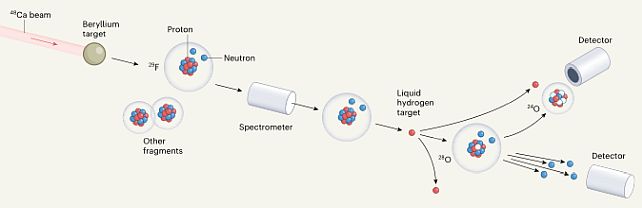
This fluorine-29 was then separated out and collided with a liquid hydrogen target to knock off a proton in an attempt to create oxygen-28.
The attempt was successful, but surprising. Both oxygen-27 and oxygen-28 are unstable, lasting for just a moment of time before decaying into oxygen-24 and 3 or 4 loose neutrons, respectively, and this is where things get interesting for oxygen-28.
Both 8 and 20 are "magic" numbers for protons and neutrons respectively, a property that suggests oxygen-28 should be stable.
The total number of each depends on how every added nucleon affects the stability of proton and neutron quotas called 'shells'.
A magic number in nuclear physics is the number of nucleons that will completely fill a shell, with each new shell distinguished from the previous by a wide energy gap.
An atomic nucleus with proton and neutron shells both containing magic numbers of each is known as doubly magic, and is expected to be especially stable.
Most of the oxygen on Earth, including the air we breathe, is a doubly magic form of oxygen, oxygen-16.
Oxygen-28 was expected for a long time to be the next doubly magic oxygen isotope after oxygen-16, but previous attempts to find it came up short.
(Interestingly, evidence that oxygen-24 might be doubly magic emerged in 2009, suggesting that 16 could be a magic number.)
The work of Kondo and his colleagues could explain why. Their findings suggest that the neutron shell had not been filled. This calls into question whether or not 20 is a magic number for neutrons.
Interestingly, it seems consistent with a phenomenon known as the island of inversion for isotopes of neon, sodium, and magnesium, where shells of 20 neutrons fail to close. This also extends to fluorine-29, and now, apparently oxygen-28.
Further understanding of the strangely unclosed neutron shell will have to wait until the researchers can probe the nucleus in an excited, higher-energy state. Other methods of formation for oxygen-28 could also be revealing, although that's a lot trickier to realize.
For now, the team's fascinating and hard-won results reveal that doubly magic nuclei could be a lot more complicated than we knew.
The research has been published in Nature.
