Physicists have managed to squeeze water molecules into a brand new state that doesn't adhere to the usual laws of solids, liquids, and gases. By trapping water into very tiny cracks, similar to those that also exist in nature, the researchers have managed to get its hydrogen and oxygen atoms to behave in very peculiar ways.
The discovery is closely linked to existing hypotheses in quantum physics – an area of science where the 'classic rulebook' of the Universe is often tossed out and ignored. The team behind the research isn't quite sure where their find will lead quite yet, but it should offer new insight into how water behaves in ultra-confined spaces.
Scientists from Oak Ridge National Laboratory forced water molecules down channels made from the mineral beryl, measuring just 5 angstroms across (about 1 ten-billionth of a metre), as Michael Byrne from Motherboard reports.
They say similar conditions are likely to be found in the natural world too, inside soils, mineral interfaces, and cell walls, for example.
Inside this molecular straightjacket (individual atoms are about 1 angstrom across), the two hydrogen atoms and one oxygen atom that make up a water molecule started to display some really weird behaviour.
Rather than being fixed, the hydrogen atoms began to appear in six different symmetric orientations at the same time, with the oxygen atom in the middle:
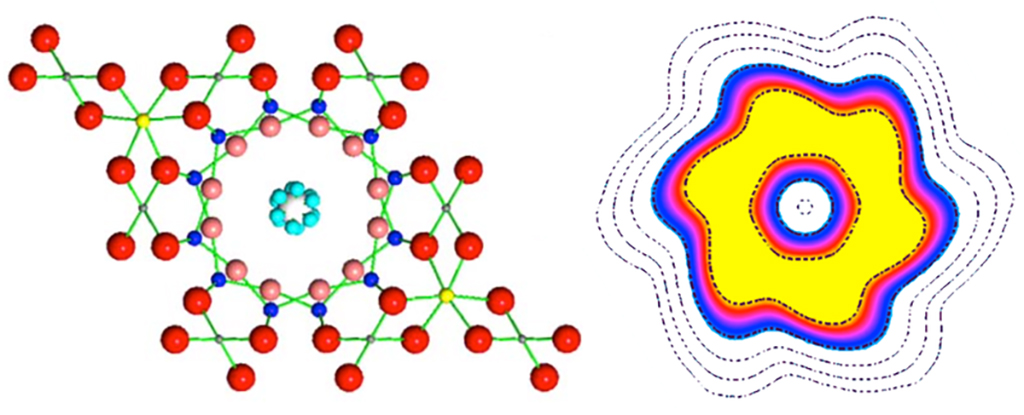 Water molecules in tunnelling mode. Credit: A. I. Kolesnikov et al.
Water molecules in tunnelling mode. Credit: A. I. Kolesnikov et al.
The six different positions match the six different walls of the hexagonal channel, the scientists say. As they tunnel, the hydrogen atoms cycle between all possible positions, and the temperature is increased as a result.
What's more, the molecule's centre of mass shifts to the central oxygen atom rather than the outlying hydrogen ones (as would be the case in a typical molecule). The newly symmetrical layout also means the molecule loses its electric dipole moment, which means the negative and positive charges in the atoms are no longer unbalanced, and in theory, it should no longer be interested in bonding with other atoms or molecules.
It's a major discovery, even if the scientists behind it aren't exactly sure what it means quite yet.
"It's one of those phenomena that only occur in quantum mechanics and has no parallel in our everyday experience," said lead researcher, Alexander Kolesnikov.
"This discovery represents a new fundamental understanding of the behaviour of water and the way water utilises energy," added team member, Lawrence Anovitz.
The next step is figuring out why this phenomenon occurs, but ultimately it should give scientists a better understanding of the thermodynamics and behaviour of water when it's in very tightly confined environments.
The team's work has been published in the journal Physical Review Letters.
