Understanding how individual neurons work in conjunction to collectively influence behaviour is a fundamental problem in neuroscience.
Previous studies have shown that neurons working together can play an important role for a variety of animal behaviours such as arm reaching in primates, song production in zebrafinches, the choice between swimming or crawling in leeches, and decision-making in mice relying on memories to navigate through a virtual environment.
Neuroscientists, however, are limited in what they can figure out about how the brain controls these behaviours, largely because conventional imaging techniques can only observe a few neurons at any one time - and animals typically need to be immobilised for them to do so.
To get a more accurate picture of neuron-teamwork, so to speak, new imaging techniques have to be developed that can observe the activity of very large clusters of neurons simultaneously. And to get the best results, this needs to be done while an animal is moving around freely, behaving as it would in nature.
Now, a team of researchers from Princeton University in the US has done just that.
In a paper published online at arXiv.org, the researchers describe a new imaging technique and instrument, which has allowed them to monitor the entire neural activity at work inside the brains of two nematode worms, Caenorhabditis elegans, which were allowed to swim around freely in a petri dish.
"No previous neurophysiological study has attained whole brain imaging with cellular resolution in a freely behaving animal," the researchers wrote.
The C. elegans was an ideal candidate for their study because it is small (about 1 mm in length), transparent, and because its nervous system, consisting of only 302 neurons, has been completely mapped.
But worms like to wiggle around, quite a lot actually, and "whole brain imaging" a constantly moving head is tricky.
In order to get the job done, the team set up two cameras to constantly monitor the worm's position and orientation (and to gauge its locomotive behaviour). They also used custom software to track the 3D position of its head (and brain). This moved a motorised platform, which allowed them to keep the head inside the field of view of a confocal microscope.
As MIT Technology Review explains, the team then used:
"a standard technique of calcium fluorescent imaging to photograph the neural activity in a thin slice of the nematode's brain. This works by genetically modifying the worms to express a fluorescent protein that glows when neurons produce calcium ions… By photographing other brain slices, the team was able to build a three-dimensional picture of the entire brain activity. And by doing this at a rate of 200 frames per second, they were able to capture five brain volumes per second as the worm moves."
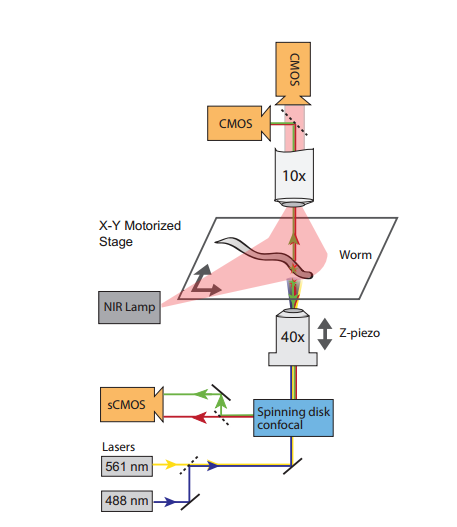 Illustration of the imaging technique (Courtesy Jeffrey Nguyen)
Illustration of the imaging technique (Courtesy Jeffrey Nguyen)
In total, the team observed activity in 78 and 68 neurons in each of the worms' brains, respectively.
And across both worms, multiple neurons showed "significant correlation" to movement behaviours such as forward and backward crawling, and turning.
According to the paper, some of the neurons they observed playing a role in behaviour were consistent with single-neuron studies, while others were newly identified.
"We believe this work represents a significant advance towards studying population dynamics of a brain-sized neural network for coding behaviour," the researchers wrote.
Source: arXiv.org, MIT Technology Review