Chemotherapy is an incredible tool for annihilating cancer cells, but our regular cells end up in the crossfire, which can result in life-altering side effects.
Chemotherapy or chemo for short is not always required for successful treatment either, but how to tell if someone requires it can be both an art and a science. Now, a new study provides clinicians with a technique that has already helped some stage II colon cancer patients avoid chemo, with no change to their clinical outcomes.
The technique employed in this new study uses a type of DNA called circulating tumor DNA (ctDNA). This is pretty self-explanatory – it's small sections of fragmented DNA from tumors that are circulating around in the blood stream. Importantly, they are not part of a tumor cell however, just the DNA from the tumor by itself.
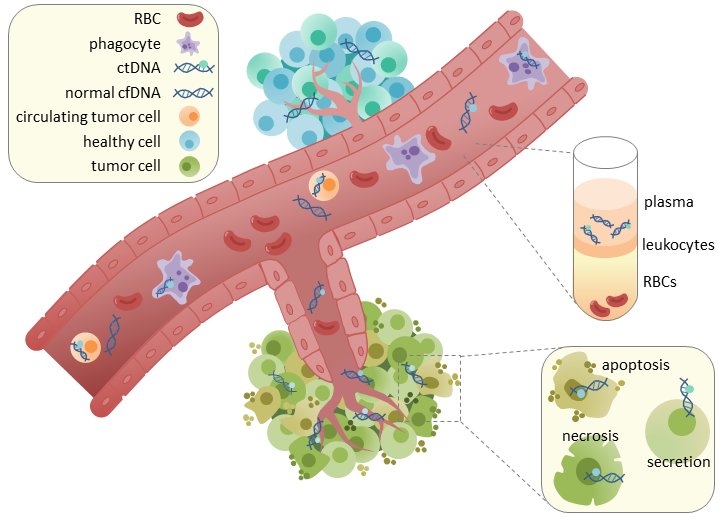 Circulating tumor DNA (ctDNA) and how it's found in blood. (Racheljunewong/Wikimedia).
Circulating tumor DNA (ctDNA) and how it's found in blood. (Racheljunewong/Wikimedia).
This work is not the first to investigate ctDNA, and researchers know that the presence of ctDNA in the bloodstream post-surgery predicts the risk of cancer recurrence.
The new study has taken this knowledge a step further, however. Looking at stage II colon cancer, it's the first clinical study showing that a ctDNA 'guided approach' after surgery can significantly benefit the patients.
"Stage II colon cancer presents a unique challenge," explains Johns Hopkins gastroenterologist Anne Marie Lennon.
"In stage I colon cancer, patients do not receive chemotherapy because their prognosis for survival is over 90 percent. The risk of discomfort and toxicities from the therapy outweigh the benefits it can provide. On the other hand, every stage III colon cancer patient currently receives chemotherapy because the risk of relapse is high."
In stage II colon cancer, the cancer has spread through the muscle layers of the colon wall, but has yet to spread to other organs. In this case, the patient will undergo surgery to remove the tumor, but then the clinician will have to make a choice on whether they also undergo chemotherapy afterwards.
We know that approximately 75 percent of people with stage II colon cancer don't require chemotherapy after surgery, but about 25 percent do. Working out which patients will benefit the most from chemo is incredibly important, and getting it wrong can be deadly.
Currently, there are a number of features a tumor might have that could prompt the doctor to book chemotherapy – for example, if the tumor looks abnormal under the microscope, or if the cancer has broken all the way through into other tissue.
But this new study has shown that this method isn't foolproof, and many cancer patients might be getting chemotherapy when they don't need it.
Between 2015 and 2019, 455 patients with type II colon cancer were recruited to the study. From this, 302 were assigned to the ctDNA guided approach, while the rest received standard care. The patients were followed up with after around 37 months, meaning the study had data on the patients for over three years.
Both the standard management and the guided treatment had similar rates of survival, and no recurrence of the cancer (92.4 vs 93.5 percent) within the study period. However, the big difference was the amount of chemotherapy that was administered. In the standard treatment group, 27.9 percent of patients underwent chemo, while in the ctDNA guided treatment group only 15.3 had to.
That's almost double the number of patients who underwent chemotherapy, for no increase in survival or decrease in tumor recurrence.
"A ctDNA-guided approach to stage II colon cancer reduced adjuvant chemotherapy use without compromising recurrence-free survival," the researchers – lead by Walter and Eliza Hall Institute gastrointestinal oncologist Jeanne Tie – wrote in their new paper.
"The low recurrence rate in ctDNA-positive patients who received chemotherapy suggests a survival benefit from adjuvant therapy."
The researchers hope ctDNA could also be a helpful indicator for how to treat other types of cancer and other stages of colon cancer, and the team is already working on early stage pancreatic and stage III colon cancer to see if ctDNA can help there too.
"We have an opportunity to change clinical practice," says biomedical engineer Joshua Cohen, one of the researchers from Johns Hopkins University School of Medicine.
"Using ctDNA to guide treatment, a stage II colon cancer patient who is negative for ctDNA has a lower chance of cancer recurrence than the average stage I colon cancer patient."
The research has been presented at the annual meeting of the American Society of Clinical Oncology and will be published in the New England Journal of Medicine.