A parasitic fungus that infects the common housefly spreads its tiny white spores in a deeply unsettling yet admittedly impressive way.
Flying its host to a high place, the hidden fungus turns the fly into an artillery of water cannons, spraying its surroundings with some of the fastest jets of liquid measured in the natural world.
Known as Entomophthora muscae, the name of this pathogenic fungus roughly translates to "destroyer of the fly", and, incidentally, that is exactly what it does.
First up, the fungus infects and manipulates the brain of its host. Then, before digesting its guts, organs, and body fat, it forces the fly to climb and settle as high as possible.
Only when the victim has officially met its end, having been eaten from the inside out, do the cracks begin to appear. Erupting from the fly's abdomen comes an artillery of fungal structures, known as conidiophores.
Like a plethora of micrometric stalks, these protruding appendages are thought to work sort of like a water pistol; each and every one is a liquid-pressurised cannon, ready to let loose on the world below.
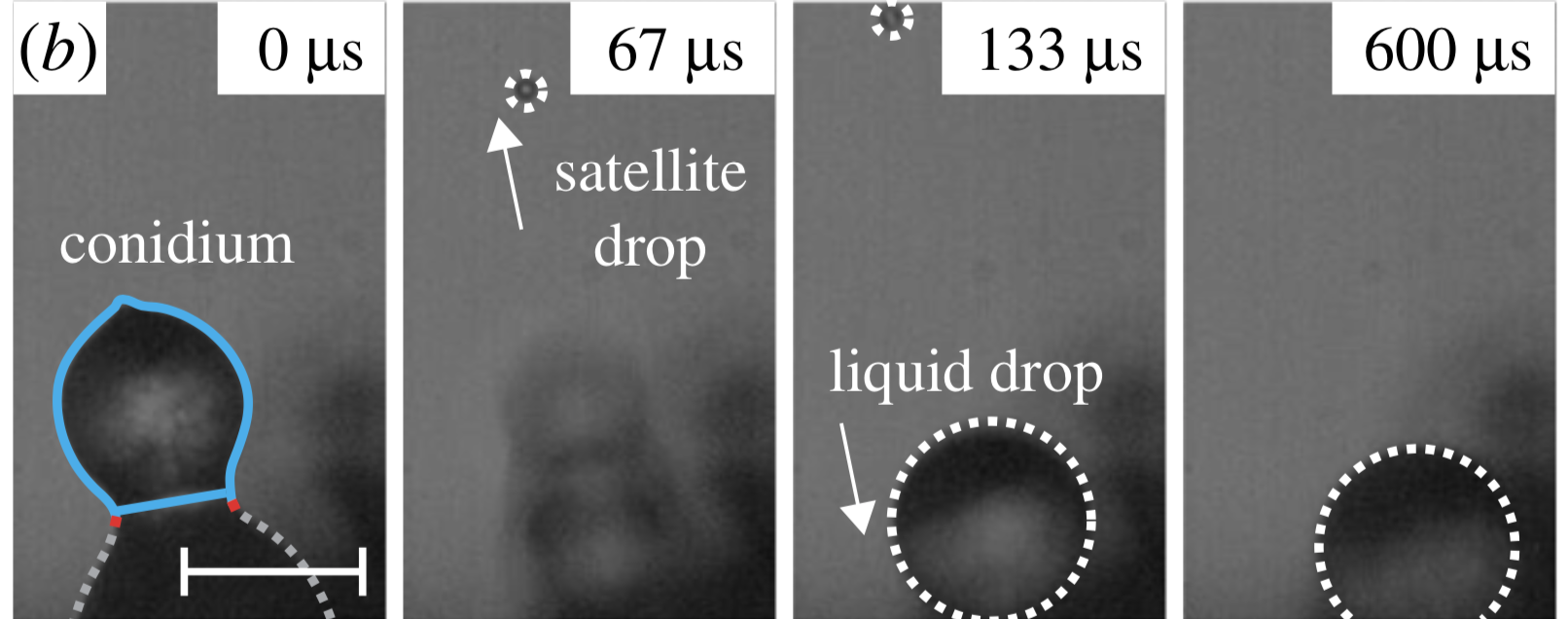 (Ruiter et al., Journal of the Royal Society Interface, 2019)
(Ruiter et al., Journal of the Royal Society Interface, 2019)
Such supremely fast cannons are seen in several types of fungi, and while they've fascinated scientists for centuries, the actual dynamics have been mostly overlooked.
In fact, the speed of E. muscae's ejection was only recently measured, coming in at an impressive 10 metres per second, which is roughly 36 kilometres per hour (22 miles per hour).
Using an ultra high-speed video camera and a tiny synthetic cannon of their own making, researchers were able to zoom in on some of the key mechanisms behind this ejection.
The cannon they designed is essentially a barrel on a miniature scale, filled with fluid and plugged with a projectile. By manipulating the tube's geometry, thickness, and elasticity as well as the projectile, the authors were able to analyse how the launch and pressurising liquid might operate in natural fungi.
The findings suggest how fungal spores are able to reach a new host, and it turns out, they are optimally sized for the absolute greatest launch distance.
While spreading tiny spores over just a few centimetres may not seem like it would take much, in reality, that distance requires very high launch speeds. Otherwise, these microscopic projectiles won't take to the wind or find a new victim, being overwhelmed by aerodynamic drag.
"Although smaller projectiles have higher ejection velocity," the authors write, "they also experience larger aerodynamics drag and we find that there is a minimum spore size ≳10μm that is able to traverse the quiescent layer (of a few millimetres) around the sporulating fly."
This means the spore itself is quite small compared to the liquid that's typically discharged along with it.
"This corroborates with the natural size E. muscae conidia (approx. 27 μm) being large enough to traverse the boundary layer but small enough (less than 40 μm) to be lifted by air currents," the authors explain.
Once it lands on a new victim, the conidiophore then forcibly discharges another conidium in the exact same way, and this is what ultimately infects the new host. This two-step 'cascade', the authors conclude, appears to substantially improve E. muscae's dispersal.
The research was published in the Journal of the Royal Society Interface.
