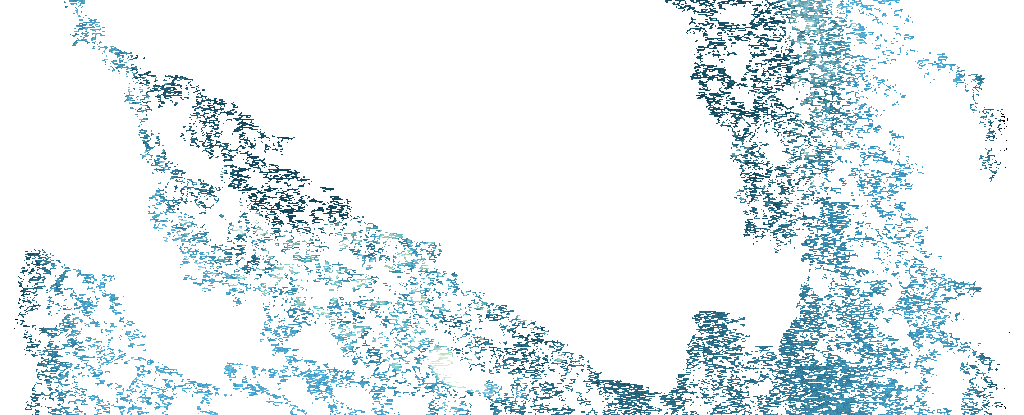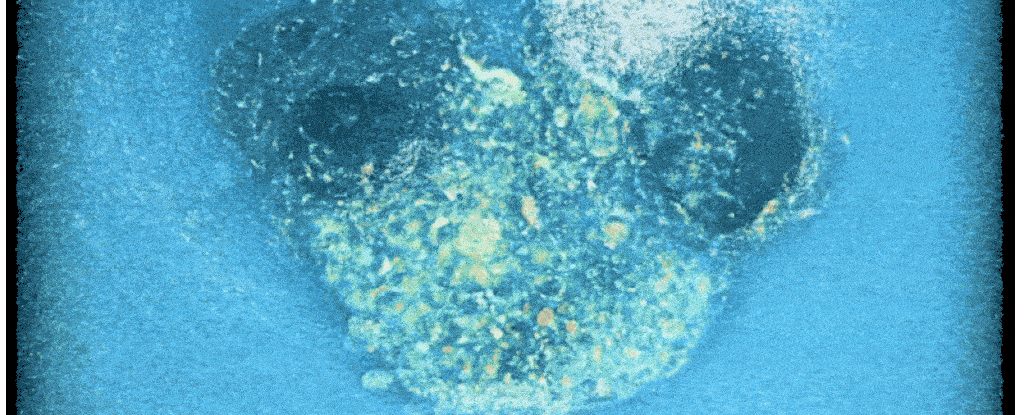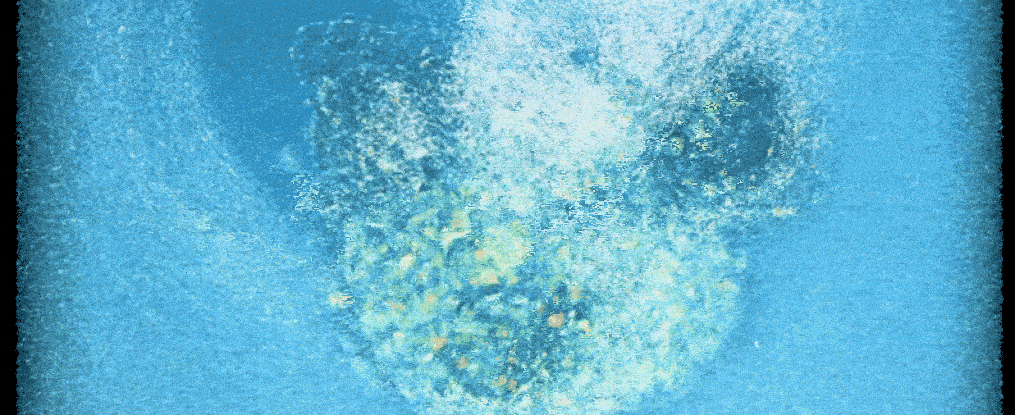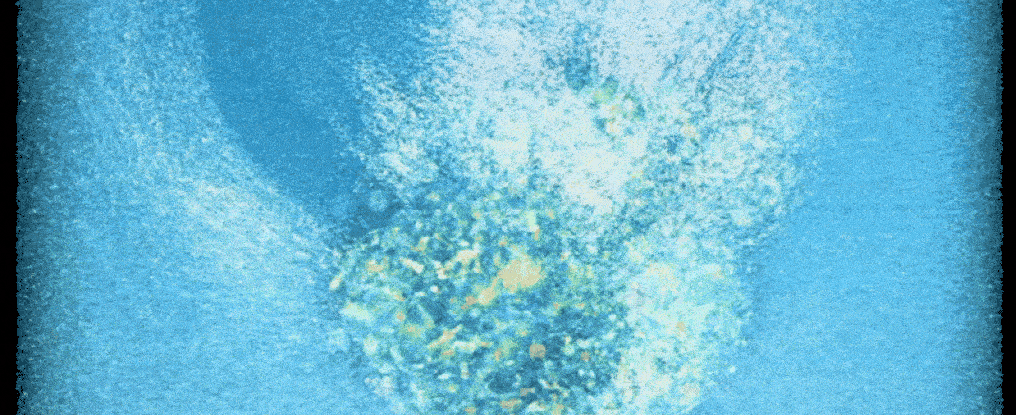
Scientists have for the first time ever filmed a crucial step that leads to new human life – cracking open a 'black box' of early development, and potentially helping with fertility treatments.
Every human alive today was once nothing more than a free-floating clump of cells looking for a suitable place to call home. Despite the odds, our embryos did it: they became one with our mother's body.
This achievement, known as implantation, occurs deep within the uterus, and until now, scientists had only taken snapshots of the phenomenon.
It takes weeks before an ultrasound can glimpse anything in the darkness.
Related: Complete 2-Week Old Human Embryo Models Grown in The Lab For The First Time
A new system now lets researchers take a closer look at the surprisingly invasive process. Time-lapse recordings show human embryos in the lab aggressively penetrating a collagen-based matrix to create a cavity for connection and further growth.

"For the first time, we've been able to watch human embryo implantation unfold dynamically," senior author and bioengineer Samuel Ojosnegros of the Barcelona Institute of Science and Technology (BIST) told ScienceAlert.
"We've opened a window into a stage of development that was previously hidden."
The experiments took place in the lab, not in a real uterus; however, the platform developed by Ojosnegros and his colleagues recreates the right structural environment and nutrients for donated embryos to implant.
This is a critical step that can easily go wrong under natural conditions. Roughly 60 percent of failed pregnancies happen during implantation or soon after, making the stage a key bottleneck to life.
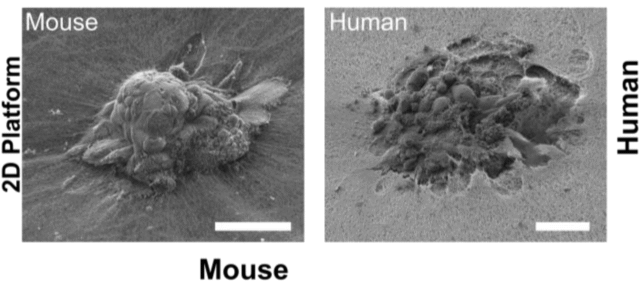
Compared to mouse embryos, which only invaded the matrix superficially, researchers found that human embryos drilled deep, fully wrapping themselves in the collagen matrix.
"Our technology lets us pinpoint where the embryo exerts force, and we found it applies significant mechanical force to implant and invade," said Ojosnegros.
"This means that mouse studies can only take us so far in understanding human implantation."

A human embryo typically implants five to six days after fertilization (when egg and sperm meet). At this stage, the embryo is a cluster of 100 to 200 cells – too small to be seen using ultrasound.
In the past, this meant that researchers could mainly watch the first five days of embryo development in the lab.
The new uterine model developed by Ojosnegros and his colleagues widens the window, extending embryo observation beyond that initial first stage. The technology can be used as a flat gel or as a droplet to observe embryo implantation in 2D or 3D.
When blastocysts are left on top of a flat gel, they can be seen binding to the collagen surface and then invading it.
When they are placed inside the droplets, meanwhile, the embryos seem to 'pull' the collagen fibers of the mother's tissues toward their center, remodeling the environment around themselves.

Lead author of the study Amélie Luise Godeau from BIST and her team speculate that the embryo is somehow connecting the maternal environment with its own tissues.
What the uterine wall might do in response to that is beyond the scope of the study. The collagen-based matrix is not made using human uterine cells, so it can only provide half the picture.

That limitation, however, also offers an advantage. The composition of the matrix can be tweaked to see how human embryos respond to different environments or compounds that might improve implantation.
"For example, through our spin-off, Serabiotics, and in collaboration with the pharmaceutical company Grifols, we've developed a protein supplement that can be used in clinics to enhance implantation rates," said Ojosnegros, the cofounder of Serabiotics.
The team of researchers is keen to keep studying embryo implantation to better understand the mysterious and critical stage of development.
The study was published in Science Advances.