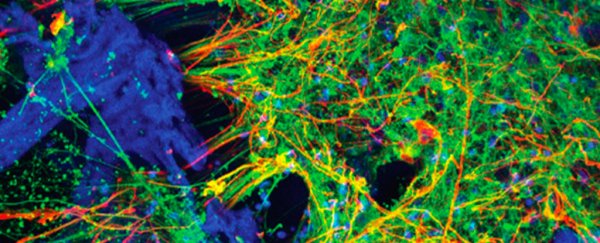
A working 3D model of human brain tissue has been grown from cultures of induced pluripotent stem cells, giving researchers even better opportunities to explore interactions between healthy and abnormal brain cells.
While 'mini-brains' have been grown under laboratory conditions for years – representing not just human brains but those of our extinct relatives – this technique takes a slightly different approach to develop an accurate, three-dimensional scaffold of functional neural tissue.
Research led by neuroscientists from Tufts University in Massachusetts has taken a new approach to building tiny brains outside of the human body based on induced pluripotent stem cells, or iPSCs; cells from around the body that have been encouraged to revert to a blank state.
"We found the right conditions to get the iPSCs to differentiate into a number of different neural subtypes, as well as astrocytes that support the growing neural networks," says biomedical engineer David L. Kaplan from Tufts.
Using stem cells to create so-called organoids of nervous tissue is itself nothing new. We've been doing it to create models of human brains for some time, studying them not only in glassware but inside animal models as well.
But there are still a number of challenges involved in the development of these blobs of tissue. Many grow into dense clusters, making it hard for oxygen to circulate and challenging to pick out individual cells while maintaining an authentic, three-dimensional arrangement.
Growing neural tissues into an accurate representation of a network of brain cells that can be studied easily, demands the perfect 'reef' for the cells to cling to, and the right surroundings to promote their differentiation into the right cell types.
Some approaches make use of tissue-like hydrogels, while others turn to porous polystyrene scaffolds. Each has its advantages, but comes with costs.
This new method mixes things up a little, creating a web-like matrix from the silk protein fibroin to space out the cells, and then immersing it in a collagen hydrogel for an authentic support structure.
"The silk-collagen scaffolds provide the right environment to produce cells with the genetic signatures and electrical signaling found in native neuronal tissues," says Kaplan.
Such a balance of architecture makes an ideal home for stem cells to settle in and develop into the rich variety of cells we'd find in an adult human brain, spacing them out without compromising on their activity.
Better still, spreading out the cells allows nutrients and oxygen to move through the tissue far better.
By creating a 'doughnut' shaped structure for the matrix, the researchers were able to make use of a central window into the developing tissue, and watch it grow in real time. Different structures in the future could be used to monitor growth in a variety of ways.
Given the difficulties and ethical challenges in studying the growth and development of both healthy and diseased human neural networks, finding better ways to analyse brain cells grown in a setting that's as natural as possible is vital for research.
These organoids are a promising step in the right direction.
"The growth of neural networks is sustained and very consistent in the 3D tissue models, whether we use cells from healthy individuals or cells from patients with Alzheimer's or Parkinson's disease," says Tufts University researcher William Cantley.
"That gives us a reliable platform to study different disease conditions and the ability to observe what happens to the cells over the long term."
Future developments are set to weave in even more cell types, creating ever more complex organoids structured to make them both an accurate representation and easy to study.
This research was published in ACS Biomaterials Science & Engineering.