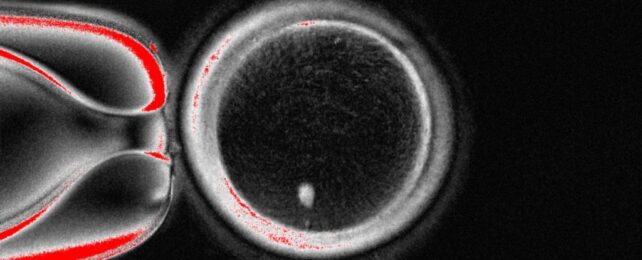
Scientists have created egg-like cells capable of fertilization using DNA from ordinary skin cells in what could be a major breakthrough for infertility research.
Using a newly developed technique to remove excess chromosomes, a team led by clinical biologist Nuria Marti-Gutierrez of Oregon Health & Science University has created human eggs that can undergo successful fertilization and start to develop into zygotes.
A clinical application for this technique is still at least 10 to 15 years away, the authors speculate, and some key challenges and ethical obstacles remain, but it's a proof of concept that may offer hope for future cases of infertility.
Related: 8 Babies Born in UK Using Radical 'Three Parent' IVF Technique
"We can harness the cellular machinery of the mature oocyte to essentially reprogram a somatic cell rather than relying on months of cell culture to produce induced pluripotent stem cells," OB/GYN and reproductive endocrinologist Paula Amato of Oregon Health & Science University told ScienceAlert.
"It theoretically saves time and potentially results in fewer genetic and epigenetic aberrations."
Infertility affects millions of people globally. It's officially defined as an inability to achieve a successful pregnancy after 12 months of trying, and there can be any number of contributing causes. Among those causes are gametes that don't function properly, whether that's the sperm, the ova, or a combination of both.
There can also be many reasons that ova don't function, from diseases such as cancer to age-related decline in oocyte quantity and quality – those are the cells that develop into eggs. One possible treatment involves a technique called in vitro gametogenesis (IVG), which involves making gametes using the patient's own genetic material. This has been achieved in mice, but not humans.
Somatic cell nuclear transfer (SCNT) is a form of IVG in which the nucleus of an egg cell is replaced with the nucleus of a somatic, or body, cell. However, it presents significant challenges of its own. One of these is extra chromosomes. A normal gamete contains one set of 23 chromosomes, half the number found in most body cells. A gamete created with SCNT has 46.
To solve this problem, the researchers developed a technique they call mitomeiosis, an artificial method that mimics the natural process of cell division.
"The nucleus (containing the DNA) of a skin cell from the intended parent is transferred into a donor oocyte (egg) that's had its nucleus removed," Amato explained.
"That reconstructed egg is then induced to extrude half of its chromosome complement to achieve haploidy (23 chromosomes). This is the process we are calling 'mitomeiosis'. The now-haploid egg is then fertilized with sperm from the other intended parent, resulting in a diploid zygote (embryo), hopefully with a normal number of chromosomes (46 – half from each parent)."
Using this technique, the researchers created 82 functional oocytes using eggs and skin from consenting donors. Then, they used donor sperm to fertilize the oocytes.
The results were mixed. Most of the oocytes stopped developing at the 4-to-10-cell stage of division. About 9 percent continued to develop into a blastocyst – a relatively low rate, but a successful first demonstration that the technique can work. The experiment was terminated on the sixth day of development, because that's the point at which a developing blastocyst is usually implanted into a patient.
The blastocysts showed signs of chromosomal abnormalities, because the ejection of additional chromosomal material during mitomeiosis was random. This presents the next challenge to be tackled.
"Unless the embryo contains a normal number of chromosomes, i.e., one from each of the 23 pairs, the embryo will not develop normally and would not result in a healthy baby," Amato said. "We are now working on ways to enhance chromosome pairing and segregation to result in a normal complement of chromosomes in the resulting embryos."
Refining the technique is likely to take at least a decade, Amato cautioned. But it's a success that demonstrates the feasibility of mitomeiosis, while laying out a clear path for further research.
"For the first time, scientists have shown that DNA from ordinary body cells can be placed into an egg, activated, and made to halve its chromosomes, mimicking the special steps that normally create eggs and sperm," says fertility specialist Ying Cheong of the University of Southampton in the UK, who was not involved in the study.
"While this is still very early laboratory work, in the future it could transform how we understand infertility and miscarriage, and perhaps one day open the door to creating egg- or sperm-like cells for those who have no other options."
The research has been published in Nature Communications.