When metal alloys are processed during manufacturing, the atoms of the combined elements are mixed together at random, according to conventional wisdom – but new research challenges this thinking, revealing hidden atomic patterns that persist.
The study is the work of researchers from the Massachusetts Institute of Technology (MIT), and it promises to open up new ways to control the properties of metals during manufacturing
Recent lab studies have identified subtle patterns in metal alloys that can be tweaked to enhance the material's properties, including mechanical strength, durability and radiation tolerance. This new study reveals in simulations how those patterns – and some new ones – emerge and linger even after intense processing.
Related: Breakthrough: Quantum Entanglement Achieved Between The Hearts of Two Atoms
"This is the first paper showing these non-equilibrium states that are retained in the metal," says MIT materials scientist Rodrigo Freitas.
"Right now, this chemical order is not something we're controlling for or paying attention to when we manufacture metals."
Understanding the new findings is a little tricky if you're not already familiar with the physics of metal alloys, but the chemical short-range order (SRO) that the researchers were looking at in this study is the arrangement that atoms fall into in metal alloys.
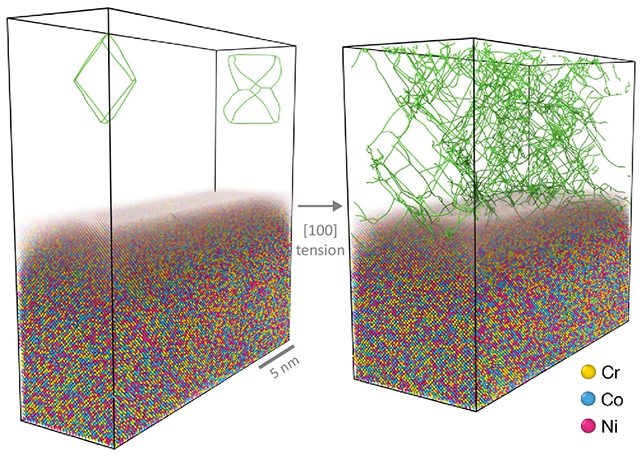
The team ran detailed computer simulations to track the interactions of millions of atoms in an alloy of chromium, cobalt, and nickel (CrCoNi) during intense deforming processes that are common in manufacturing – specifically, quick cooling and extensive stretching.
First, the researchers saw atomic patterns that were familiar but that wouldn't be expected to still be intact after such rapid deformations. Second, they found completely new patterns, which they're calling "far-from-equilibrium states".
Crucial to the survival of these states, the models showed, are the defects (or dislocations) that form in the crystal structure of metals as they are heated and cooled, or stretched. They're almost like atomic-level scribbles, and they help metal take the strain exerted upon it.
Before now, it was assumed that deformations and the subsequent movement of defects pretty much wiped out SRO, but the models run by the researchers showed that atoms actually shuffle around with some predictability.
"These defects have chemical preferences that guide how they move. They look for low energy pathways, so given a choice between breaking chemical bonds, they tend to break the weakest bonds, and it's not completely random," says Freitas.
"This is very exciting because it's a non-equilibrium state: it's not something you'd see naturally occurring in materials."
In other words, these newly discovered patterns are only brought about because of manufacturing processes. They will then, in turn, affect the various properties of the metal – which is something future studies can start to look at in more detail.
While the physics involved here is quite high-level, the implications are that we can fine-tune the properties of metal alloys in ways that haven't been thought about before, for everything from nuclear reactors to spacecraft – because of the way that their atoms refuse to be completely shuffled up.
"The conclusion is: you can never completely randomize the atoms in a metal. It doesn't matter how you process it," says Freitas."
"The fact that you cannot completely mix something together, people didn't see that coming."
The research has been published in Nature Communications.